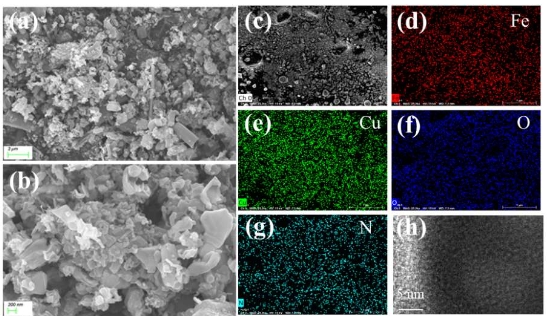
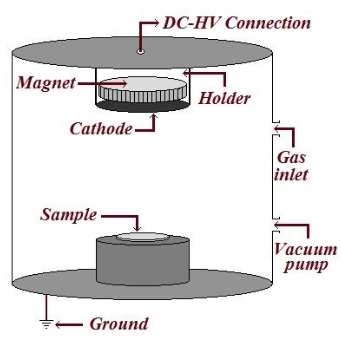
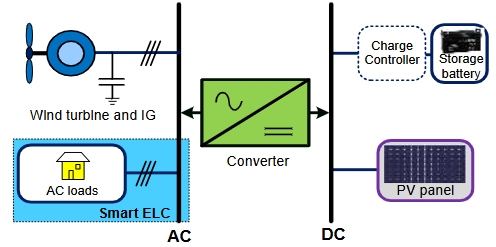
Battery and/or supercapacitor?—On the merger of two electrochemical storage system families
Abstract
Similarities and analogies between materials, structures, operating and construction principles of secondary batteries and supercapacitors and their electrodes are presented, named, and reviewed in context. On the material level, several materials used both in batteries and supercapacitors are addressed, and implications from observations made in one application for the other are highlighted. On the electrode level, a continuous change of architectural details is observed when going from an electrode with high charge storage capability to an electrode supporting high currents is detected; again, this overlap provides instructive ideas for both fields. On the cell level, combinations of electrodes from both fields yielding hybrid devices are an obvious outcome again, with implications for both fields. Ideas and suggestions for further research and development based on a deeper exchange between both families are developed.
References
[1]Wu Y, Holze R. Electrochemical Energy Conversion and Storage. VCH-WILEY; 2022.
[2]Kurzweil P, Dietlmeier OK. Elektrochemische Speicher. Springer Vieweg; 2015.
[3]Ge Y, Xie X, Roscher J, et al. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. Journal of Solid State Electrochemistry. 2020; 24(11-12): 3215-3230. doi: 10.1007/s10008-020-04804-x
[4]Becker HI. U.S. Patent US2800616, 23 July 1957.
[5]Rightmire RA. U.S. Patent US3288641, 29 November 1966.
[6]Wang G, Zhang L, Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev. 2012; 41(2): 797-828. doi: 10.1039/c1cs15060j
[7]Dubal DP, Wu Y, Holze R. Supercapacitors as fast storage systems for electric energy. Bunsen-Magazin. 2015; 17: 216-227.
[8]Vangari M, Pryor T, Jiang L. Supercapacitors: Review of Materials and Fabrication Methods. Journal of Energy Engineering. 2013; 139: 72-92.
[9]Vol’fkovich YM, Serdyuk TM. Electrochemical capacitors. Russian Journal of Electrochemistry. 2002; 38: 935-958.
[10]Burke A. Ultracapacitors: Why, how, and where is the technology. Journal of Power Sources. 2000; 91: 37-50.
[11]Shukla AK, Sampath S, Vijayamohanan K. Electrochemical supercapacitors: Energy storage beyond batteries. Current Science. 2000; 79: 1656-1661.
[12]Guan L, Yu L, Chen GZ. Capacitive and non-capacitive faradaic charge storage. Electrochimica Acta. 2016; 206: 464-478. doi: 10.1016/j.electacta.2016.01.213
[13]Dubal DP, Wu YP, Holze R. Supercapacitors: from the Leyden jar to electric busses. ChemTexts. 2016; 2(3). doi: 10.1007/s40828-016-0032-6
[14]Conway BE. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications. Springer; 1999.
[15]Faggioli E, Rena P, Danel V, et al. Supercapacitors for the energy management of electric vehicles. Journal of Power Sources. 1999; 84: 261-269.
[16]Nguyen T, Montemor MF. Redox active materials for metal compound based hybrid electrochemical energy storage: a perspective view. Applied Surface Science. 2017; 422: 492-497. doi: 10.1016/j.apsusc.2017.06.008
[17]Baptista JM, Sagu JS, KG UW, et al. State-of-the-art materials for high power and high energy supercapacitors: Performance metrics and obstacles for the transition from lab to industrial scale – A critical approach. Chemical Engineering Journal. 2019; 374: 1153-1179. doi: 10.1016/j.cej.2019.05.207
[18]Electrochemical Supercapacitors for Energy Storage and Delivery—Fundamentals and Applications. CRC Press; 2013.
[19]Electrochemical Capacitors. In: Materials Research Foundations. Materials Research Forum LLC; 2018.
[20]Stevic Z. Supercapacitor Design and Applications. ExLi4EvA; 2016.
[21]Beguin F, Frackowiak E. Supercapacitors. Wiley-VCH; 2013.
[22]Miller JM. Ultracapacitor Applications. The Institution of Engineering and Technology; 2011.
[23]Wu Y, Holze R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem Energy Technol. 2021; 7: 1-37.
[24]Gür TM. Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy & Environmental Science. 2018; 11: 2696-2767.
[25]Frequently the term electrolyte meaning in most cases electrolyte solution is used. In: Solid electrolytes and ionic liquids are further options.
[26]Holze R. From current peaks to waves and capacitive currents—on the origins of capacitor-like electrode behavior. Journal of Solid State Electrochemistry. 2016; 21(9): 2601-2607. doi: 10.1007/s10008-016-3483-1
[27]Banerjee A, Ramasesha SK, Shukla AK. A photovoltaic stand-alone lighting system with polymeric-silica-gel-electrolyte-based substrate-integrated lead-carbon hybrid ultracapacitors. Electrochemical Energy Technology. 2015; 1(1). doi: 10.1515/eetech-2015-0001
[28]Dubal DP, Ayyad O, Ruiz V, et al. Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chemical Society Reviews. 2015; 44(7): 1777-1790. doi: 10.1039/c4cs00266k
[29]Zuo W, Li R, Zhou C, et al. Battery‐Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Advanced Science. 2017; 4(7). doi: 10.1002/advs.201600539
[30]Krishnan SG, Harilal M, Pal B, et al. Improving the symmetry of asymmetric supercapacitors using battery-type positive electrodes and activated carbon negative electrodes by mass and charge balance. Journal of Electroanalytical Chemistry. 2017; 805: 126-132. doi: 10.1016/j.jelechem.2017.10.029
[31]Cericola D, Kötz R. Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits. Electrochimica Acta. 2012; 72: 1-17. doi: 10.1016/j.electacta.2012.03.151
[32]Xie J, Yang P, Wang Y, et al. Puzzles and confusions in supercapacitor and battery: Theory and solutions. Journal of Power Sources. 2018; 401: 213-223. doi: 10.1016/j.jpowsour.2018.08.090
[33]Yu L, Chen GZ. Redox electrode materials for supercapatteries. Journal of Power Sources. 2016; 326: 604-612. doi: 10.1016/j.jpowsour.2016.04.095
[34]Akinwolemiwa B, Peng C, Chen GZ. Redox Electrolytes in Supercapacitors. Journal of The Electrochemical Society. 2015; 162(5): A5054-A5059. doi: 10.1149/2.0111505jes
[35]Chen GZ. Supercapacitor and supercapattery as emerging electrochemical energy stores. International Materials Reviews. 2016; 62(4): 173-202. doi: 10.1080/09506608.2016.1240914
[36]Conway BE. Electrochemical Capacitors. The Electrochemical Society Inc; 1996.
[37]Conway BE, Birss V, Wojtowicz J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. Journal of Power Sources. 1997; 66: 1-14.
[38]Simon P, Gogotsi Y, Dunn B. Where Do Batteries End and Supercapacitors Begin? Science. 2014; 343(6176): 1210-1211. doi: 10.1126/science.1249625
[39]Gogotsi Y, Penner RM. Energy Storage in Nanomaterials – Capacitive, Pseudocapacitive, or Battery-like? ACS Nano. 2018; 12(3): 2081-2083. doi: 10.1021/acsnano.8b01914
[40]Kim ND, Buchholz DB, Casillas G, et al. Hierarchical Design for Fabricating Cost‐Effective High Performance Supercapacitors. Advanced Functional Materials. 2014; 24(26): 4186-4194. doi: 10.1002/adfm.201304130
[41]Holze R. Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry. Springer-Verlag; 2007.
[42]Conway BE, Gileadi E. Kinetic theory of pseudo-capacitance and electrode reactions at appreciable surface coverage. Transactions of the Faraday Society. 1962; 58: 2493. doi: 10.1039/tf9625802493
[43]Conway BE. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. Journal of The Electrochemical Society. 1991; 138(6): 1539-1548. doi: 10.1149/1.2085829
[44]Dubal DP, Holze R. Synthesis, properties, and performance of nanostructured metal oxides for supercapacitors. Pure and Applied Chemistry. 2014; 86(5): 611-632. doi: 10.1515/pac-2013-1021
[45]Dubal DP, Chodankar NR, Gomez-Romero P, et al. Fundamentals of Binary Metal Oxide–Based Supercapacitors. In: Dubal DP, Gomez-Romero P (editors). Metal Oxides in Supercapacitors. Elsevier; 2017. pp. 79-98. doi: 10.1016/b978-0-12-810464-4.00004-8
[46]Xie X, Holze R. Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries. 2022; 8(10): 146. doi: 10.3390/batteries8100146
[47]Xie X, Holze R. Meaning and Determination of Electrode Surface Area. Available online: https://encyclopedia.pub/entry/41569 (accessed on 20 November 2023).
[48]Brousse T, Bélanger D, Long JW. To Be or Not To Be Pseudocapacitive? Journal of The Electrochemical Society. 2015; 162(5): A5185-A5189. doi: 10.1149/2.0201505jes
[49]Jiang Y, Liu J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy & Environmental Materials. 2019; 2(1): 30-37. doi: 10.1002/eem2.12028
[50]Sarangapani S, Tilak BV, Chen CP. Materials for Electrochemical Capacitors: Theoretical and Experimental Constraints. Journal of the Electrochemical Society. 1996; 143(11). doi: 10.1149/1.1837291
[51]Sarangapani S, Tilak BV, Chen C ‐P. Materials for Electrochemical Capacitors: Theoretical and Experimental Constraints. Journal of The Electrochemical Society. 1996; 143(11): 3791-3799. doi: 10.1149/1.1837291
[52]Grahame DC. Properties of the Electrical Double Layer at a Mercury Surface. I. Methods of Measurement and Interpretation of Results. Journal of the American Chemical Society. 1941; 63(5): 1207-1215. doi: 10.1021/ja01850a014
[53]Burke LD, Murphy OJ. Cyclic voltammetry as a technique for determining the surface area of RuO2 electrodes. Journal of Electroanalytical Chemistry. 1979; 96: 19-27.
[54]Ardizzone S, Fregonara G, Trasatti S. “Inner” and “outer” active surface of RuO2 electrodes. Electrochimica Acta. 1990; 35: 263-269.
[55]Chen GZ. Understanding supercapacitors based on nano-hybrid materials with interfacial conjugation. Progress in Natural Science: Materials International. 2013; 23(3): 245-255. doi: 10.1016/j.pnsc.2013.04.001
[56]Reddy TB. Linden’s Handbook of Batteries. MacGraw-Hill; 2011.
[57]Daniel C, Besenhard JO. Handbook of Battery Materials. WILEY-VCH; 2011.
[58]Augustyn V, Simon P, Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy & Environmental Science. 2014; 7(5): 1597. doi: 10.1039/c3ee44164d
[59]Tian ZW, Dong QF, Zheng MS, Lin ZG. U.S. Patent US20090190286, 30 July 2009.
[60]Holze R. Supercapacitors as energy storage (German). Nachr Chem. 2017; 65: 333-338.
[61]Beck F, Euler KJ. Electrochemical energy storage (German). VDE-Verlag GmbH; 1984.
[62]Lou S, Cheng X, Gao J, et al. Pseudocapacitive Li+ intercalation in porous Ti2Nb10O29 nanospheres enables ultra-fast lithium storage. Energy Storage Materials. 2018; 11: 57-66. doi: 10.1016/j.ensm.2017.09.012
[63]Volkov AI, Dubal DP, Holze R, Wu Y. Mixed metal chalcogenides as active masses for supercapacitor electrodes – A review. Advanced Energy Materials.
[64]Placke T, Heckmann A, Schmuch R, et al. Perspective on Performance, Cost, and Technical Challenges for Practical Dual-Ion Batteries. Joule. 2018; 2(12): 2528-2550. doi: 10.1016/j.joule.2018.09.003
[65]Placke T, Fromm O, Lux SF, et al. Reversible Intercalation of Bis(trifluoromethanesulfonyl)imide Anions from an Ionic Liquid Electrolyte into Graphite for High Performance Dual-Ion Cells. Journal of The Electrochemical Society. 2012; 159(11): A1755-A1765. doi: 10.1149/2.011211jes
[66]Heidrich B, Heckmann A, Beltrop K, et al. Unravelling charge/discharge and capacity fading mechanisms in dual-graphite battery cells using an electron inventory model. Energy Storage Materials. 2019; 21: 414-426. doi: 10.1016/j.ensm.2019.05.031
[67]Heckmann A, Thienenkamp J, Beltrop K, et al. Towards high-performance dual-graphite batteries using highly concentrated organic electrolytes. Electrochimica Acta. 2018; 260: 514-525. doi: 10.1016/j.electacta.2017.12.099
[68]Kravchyk KV, Kovalenko MV. Rechargeable Dual‐Ion Batteries with Graphite as a Cathode: Key Challenges and Opportunities. Advanced Energy Materials. 2019; 9(35). doi: 10.1002/aenm.201901749
[69]Walter M, Kovalenko MV, Kravchyk KV. Challenges and benefits of post-lithium-ion batteries. New Journal of Chemistry. 2020; 44(5): 1677-1683. doi: 10.1039/c9nj05682c
[70]Sui Y, Liu C, Masse RC, et al. Dual-ion batteries: The emerging alternative rechargeable batteries. Energy Storage Materials. 2020; 25: 1-32. doi: 10.1016/j.ensm.2019.11.003
[71]Wang X, Han X, Lim M, et al. Nickel Cobalt Oxide-Single Wall Carbon Nanotube Composite Material for Superior Cycling Stability and High-Performance Supercapacitor Application. The Journal of Physical Chemistry C. 2012; 116(23): 12448-12454. doi: 10.1021/jp3028353
[72]Dubal DP, Chen X, Wu Y, Holze R. Conducting Polymers for Supercapacitors. In: Gupta RK (editor). Conducting Polymers for Advanced Energy Applications. CRC Press; 2021.
[73]Borenstein A, Hanna O, Attias R, et al. Carbon-based composite materials for supercapacitor electrodes: a review. Journal of Materials Chemistry A. 2017; 5(25): 12653-12672. doi: 10.1039/c7ta00863e
[74]Holze R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes—An Introduction. Polymers. 2020; 12(8): 1835. doi: 10.3390/polym12081835
[75]Holze R. Conjugated Molecules and Polymers in Secondary Batteries: A Perspective. Molecules. 2022; 27(2): 546. doi: 10.3390/molecules27020546
[76]Kondratiev VV, Holze R. Intrinsically conducting polymers and their combinations with redox-active molecules for rechargeable battery electrodes: An update. Chemical Papers. 2021; 75(10): 4981-5007. doi: 10.1007/s11696-021-01529-7
[77]Holze R, Wu YP. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochimica Acta. 2014; 122: 93-107. doi: 10.1016/j.electacta.2013.08.100
[78]Fong KD, Wang T, Smoukov SK. Multidimensional performance optimization of conducting polymer-based supercapacitor electrodes. Sustainable Energy & Fuels. 2017; 1(9): 1857-1874. doi: 10.1039/c7se00339k
[79]Lu Y, Zhang Q, Li L, et al. Design Strategies toward Enhancing the Performance of Organic Electrode Materials in Metal-Ion Batteries. Chem. 2018; 4(12): 2786-2813. doi: 10.1016/j.chempr.2018.09.005
[80]Huang T, Long M, Xiao J, et al. Recent research on emerging organic electrode materials for energy storage. Energy Materials. 2022; 1(1): 100009. doi: 10.20517/energymater.2021.09
[81]Jia X, Ge Y, Shao L, et al. Tunable Conducting Polymers: Toward Sustainable and Versatile Batteries. ACS Sustainable Chemistry & Engineering. 2019; 7(17): 14321-14340. doi: 10.1021/acssuschemeng.9b02315
[82]Espinoza-Acosta JL, Torres-Chávez PI, Olmedo-Martínez JL, et al. Lignin in storage and renewable energy applications: A review. Journal of Energy Chemistry. 2018; 27(5): 1422-1438. doi: 10.1016/j.jechem.2018.02.015
[83]Chaleawlert‐umpon S, Berthold T, Wang X, et al. Kraft Lignin as Electrode Material for Sustainable Electrochemical Energy Storage. Advanced Materials Interfaces. 2017; 4(23). doi: 10.1002/admi.201700698
[84]Lahiri A, Yang L, Höfft O, et al. Biodegradable Zn-ion battery with a lignin composite electrode and bio-ionic liquid based electrolyte: possible in situ energy generation by lignin electrocatalysis. Materials Advances. 2021; 2(8): 2676-2683. doi: 10.1039/d0ma00954g
[85]Zhu J, Yan C, Zhang X, et al. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Progress in Energy and Combustion Science. 2020; 76: 100788. doi: 10.1016/j.pecs.2019.100788
[86]Gnedenkov SV, Opra DP, Sinebryukhov SL, et al. Hydrolysis lignin-based organic electrode material for primary lithium batteries. Journal of Solid State Electrochemistry. 2013; 17(10): 2611-2621. doi: 10.1007/s10008-013-2136-x
[87]Gnedenkov SV, Opra DP, Sinebryukhov SL, et al. Hydrolysis lignin: Electrochemical properties of the organic cathode material for primary lithium battery. Journal of Industrial and Engineering Chemistry. 2014; 20(3): 903-910. doi: 10.1016/j.jiec.2013.06.021
[88]Gnedenkova SV, Opra DP, Zemnukhova LA, et al. Electrochemical performance of Klason lignin as a low-cost cathode-active material for primary lithium battery. Journal of Energy Chemistry. 2015; 24: 346-352.
[89]Larcher D, Tarascon JM. Towards greener and more sustainable batteries for electrical energy storage. Nature Chemistry. 2014; 7(1): 19-29. doi: 10.1038/nchem.2085
[90]Liu L, Solin N, Inganäs O. Bio Based Batteries. Advanced Energy Materials. 2021; 11(43). doi: 10.1002/aenm.202003713
[91]Wang M, Xu YX. Design and construction of three-dimensional graphene/conducting polymer for supercapacitors. Chinese Chemical Letters. 2016; 27(8): 1437-1444. doi: 10.1016/j.cclet.2016.06.048
[92]Wang J, Li X, Du X, et al. Polypyrrole composites with carbon materials for supercapacitors. Chemical Papers. 2016; 71(2): 293-316. doi: 10.1007/s11696-016-0048-9
[93]Sebastian J, Samuel JM. Recent advances in the applications of substituted polyanilines and their blends and composites. Polymer Bulletin. 2019; 77(12): 6641-6669. doi: 10.1007/s00289-019-03081-7
[94]Eftekhari A, Li L, Yang Y. Polyaniline supercapacitors. Journal of Power Sources. 2017; 347: 86-107. doi: 10.1016/j.jpowsour.2017.02.054
[95]Huang Z, Li L, Wang Y, et al. Polyaniline/graphene nanocomposites towards high-performance supercapacitors: A review. Composites Communications. 2018; 8: 83-91. doi: 10.1016/j.coco.2017.11.005
[96]Chauhan NPS, Mozafari M, Chundawat NS, et al. High-performance supercapacitors based on polyaniline–graphene nanocomposites: Some approaches, challenges and opportunities. Journal of Industrial and Engineering Chemistry. 2016; 36: 13-29. doi: 10.1016/j.jiec.2016.03.003
[97]Fu L, Qu Q, Holze R, et al. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: the best of both worlds? Journal of Materials Chemistry A. 2019; 7(25): 14937-14970. doi: 10.1039/c8ta10587a
[98]Dubal DP, Kim JG, Kim Y, et al. Supercapacitors Based on Flexible Substrates: An Overview. Energy Technology. 2014; 2(4): 325-341. doi: 10.1002/ente.201300144
[99]Ge Y, Liu Z, Wu Y, et al. On the utilization of supercapacitor electrode materials. Electrochimica Acta. 2021; 366: 137390. doi: 10.1016/j.electacta.2020.137390
[100]Kondratiev V, Holze R. Intrinsically Conducting Polymer Binders for Battery Electrodes. Encyclopedia. 2022; 2(4): 1753-1762. doi: 10.3390/encyclopedia2040120
[101]Benoit C, Demeter D, Bélanger D, et al. A Redox‐Active Binder for Electrochemical Capacitor Electrodes. Angewandte Chemie International Edition. 2016; 55(17): 5318-5321. doi: 10.1002/anie.201601395
[102]Van Hoang H, Holze R. Electrochemical Synthesis of Polyaniline/Montmorillonite Nanocomposites and Their Characterization. Chemistry of Materials. 2006; 18(7): 1976-1980. doi: 10.1021/cm052707w
[103]Akinwolemiwa B, Wei C, Chen GZ. Mechanisms and Designs of Asymmetrical Electrochemical Capacitors. Electrochimica Acta. 2017; 247: 344-357. doi: 10.1016/j.electacta.2017.06.088
[104]Guidelli R, Schmickler W. Electrosorption Valency and Partial Charge Transfer. In: Conway BE, Vayenas CG, White RE, Gamboa-Adelco ME (editors). Modern Aspects of Electrochemistry. Springer; 2005. doi: 10.1007/0-387-25838-8_3
[105]Ragoisha GA, Aniskevich YM. False capacitance of supercapacitors. Available online: https://arxiv.org/abs/1604.08154 (accessed on 12 December 2022).
[106]Bandeira MCE, Holze R. Impedance measurements at thin polyaniline films – the influence of film morphology. Microchimica Acta. 2006; 156(1-2): 125-131. doi: 10.1007/s00604-006-0586-x
[107]Ko JS, Lai CH, Long JW, et al. Differentiating Double-Layer, Psuedocapacitance, and Battery-like Mechanisms by Analyzing Impedance Measurements in Three Dimensions. ACS Applied Materials & Interfaces. 2020; 12(12): 14071-14078. doi: 10.1021/acsami.0c02020
[108]Gosser Jr. DK. Cyclic Voltammetry. VCH; 1993.
[109]Liu T ‐C., Pell WG, Conway BE, Roberson SL. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors: Comparison with Ruthenium Oxide. Journal of The Electrochemical Society. 1998; 145(6): 1882-1888. doi: 10.1149/1.1838571
[110]Lindström H, Södergren S, Solbrand A, et al. Li+ Ion Insertion in TiO2 (Anatase). 2. Voltammetry on Nanoporous Films. The Journal of Physical Chemistry B. 1997; 101(39): 7717-7722. doi: 10.1021/jp970490q
[111]Opitz M, Yue J, Wallauer J, et al. Mechanisms of Charge Storage in Nanoparticulate TiO2 and Li4Ti5O12 Anodes: New Insights from Scan rate-dependent Cyclic Voltammetry. Electrochimica Acta. 2015; 168: 125-132. doi: 10.1016/j.electacta.2015.03.186
[112]Forghani M, Donne SW. Method Comparison for Deconvoluting Capacitive and Pseudo-Capacitive Contributions to Electrochemical Capacitor Electrode Behavior. Journal of The Electrochemical Society. 2018; 165(3): A664-A673. doi: 10.1149/2.0931803jes
[113]Dupont MF, Donne SW. Faradaic and Non-Faradaic Contributions to the Power and Energy Characteristics of Electrolytic Manganese Dioxide for Electrochemical Capacitors. Journal of The Electrochemical Society. 2016; 163(6): A888-A897. doi: 10.1149/2.0401606jes
[114]Hall PJ, Mirzaeian M, Fletcher SI, et al. Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy & Environmental Science. 2010; 3(9): 1238. doi: 10.1039/c0ee00004c
[115]Wang W, Guo S, Lee I, et al. Hydrous Ruthenium Oxide Nanoparticles Anchored to Graphene and Carbon Nanotube Hybrid Foam for Supercapacitors. Scientific Reports. 2014; 4(1). doi: 10.1038/srep04452
[116]Chodankar NR, Pham HD, Nanjundan AK, et al. True Meaning of Pseudocapacitors and Their Performance Metrics: Asymmetric versus Hybrid Supercapacitors. Small. 2020; 16(37). doi: 10.1002/smll.202002806
[117]Pavlov D. Essentials of Lead-Acid Batteries. SAEST; 2006.
[118]Yu N, Gao L. Electrodeposited PbO2 thin film on Ti electrode for application in hybrid supercapacitor. Electrochemistry Communications. 2009; 11(1): 220-222. doi: 10.1016/j.elecom.2008.11.013
[119]Lam LT, Louey R. Development of ultra-battery for hybrid-electric vehicle applications. Journal of Power Sources. 2006; 158(2): 1140-1148. doi: 10.1016/j.jpowsour.2006.03.022
[120]Akinwolemiwa B, Chen G. Fundamental Consideration for Electrochemical Engineering of Supercapattery. Journal of the Brazilian Chemical Society. 2018. doi: 10.21577/0103-5053.20180010
[121]Yu L, Chen GZ. Supercapatteries as High-Performance Electrochemical Energy Storage Devices. Electrochemical Energy Reviews. 2020; 3(2): 271-285. doi: 10.1007/s41918-020-00063-6
[122]Chen GZ. Supercapacitor and supercapattery as emerging electrochemical energy stores. International Materials Reviews. 2016; 62(4): 173-202. doi: 10.1080/09506608.2016.1240914
[123]Chen GZ. Supercapattery: Merit merge of capacitive and Nernstian charge storage mechanisms. Current Opinion in Electrochemistry. 2020; 21: 358-367. doi: 10.1016/j.coelec.2020.04.002
[124]Laheäär A, Przygocki P, Abbas Q, et al. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochemistry Communications. 2015; 60: 21-25. doi: 10.1016/j.elecom.2015.07.022
[125]Rufer A, Barrade P. A supercapacitor-based energy-storage system for elevators with soft commutated interface. IEEE Transactions on Industry Applications. 2002; 38(5): 1151-1159. doi: 10.1109/tia.2002.803021
Copyright (c) 2024 Yuping Wu, Rudolf Holze

This work is licensed under a Creative Commons Attribution 4.0 International License.