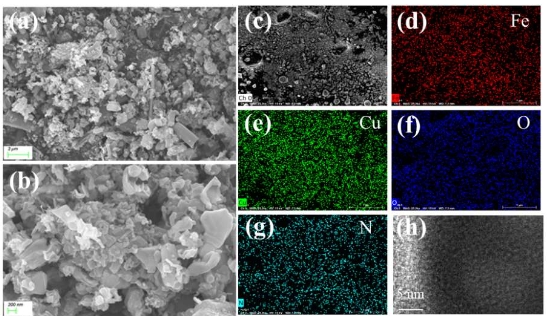
Nitrided copper-iron composite oxides derived from layered double hydroxides for enhanced carbon dioxide electroreduction to methane and formic acid
Abstract
The reduction of carbon dioxide into valuable chemical products is a promising solution to address carbon balance and energy issues. Herein, amorphous nitrided copper-iron oxides are prepared by gas-phase nitriding of CuFe-layered double hydroxide precursors with urea as a nitrogen source. The obtained materials show high activity for CO2 electroreduction to methane and formic acid, achieving a total Faraday efficiency of 74.7% at −0.7 V vs. RHE and exhibiting continuous 10 h durability in the H-cell. The uniformly distributed Cu+ sites act as active sites by losing electrons to activate CO2. During the CO2 electroreduction, CO2 is converted to *COOH via proton-electron coupling; *COOH combines directly with a proton in solution to produce the HCOOH product; and the other part of *COOH undergoes a protonated dehydration process to form the *CHO intermediate, which dehydrates again to form CH4. This study provides a new approach for designing CO2 electroreduction catalysts.
References
[1]Birdja YY, Pérez-Gallent E, Figueiredo MC, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nature Energy. 2019, 4(9): 732-745. doi: 10.1038/s41560-019-0450-y
[2]Zhang Y, Guo SX, Zhang X, et al. Mechanistic understanding of the electrocatalytic CO2 reduction reaction – New developments based on advanced instrumental techniques. Nano Today. 2020, 31: 100835. doi: 10.1016/j.nantod.2019.100835
[3]Chernyak SA, Ivanov AS, Maksimov SV, et al. Fischer-Tropsch synthesis over carbon-encapsulated cobalt and iron nanoparticles embedded in 3D-framework of carbon nanotubes. Journal of Catalysis. 2020, 389: 270-284. doi: 10.1016/j.jcat.2020.06.011
[4]Gong W, Ye RP, Ding J, et al. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission. Applied Catalysis B: Environmental. 2020, 278: 119302. doi: 10.1016/j.apcatb.2020.119302
[5]Nitopi S, Bertheussen E, Scott SB, et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chemical Reviews. 2019, 119(12): 7610-7672. doi: 10.1021/acs.chemrev.8b00705
[6]Ding P, Zhao H, Li T, et al. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. Journal of Materials Chemistry A. 2020, 8(42), 21947-21960. doi: 10.1039/D0TA08393C.
[7]Xu C, Dong Y, Zhao H, et al. CO2 Conversion Toward Real‐World Applications: Electrocatalysis versus CO2 Batteries. Advanced Functional Materials. 2023, 33(32). doi: 10.1002/adfm.202300926
[8]Plankensteiner N, Rondou N, Blom MJW, et al. Competitive enhancement of CO2 reduction reactions versus hydrogen evolution for high surface area electrodes: A comparative study for Cu and Ag nanomesh. Electrochimica Acta. 2024, 474: 143495. doi: 10.1016/j.electacta.2023.143495
[9]Pourebrahimi S, Pirooz M, Ahmadi S, et al. Nanoengineering of metal-based electrocatalysts for carbon dioxide (CO2) reduction: A critical review. Materials Today Physics. 2023, 38: 101250. doi: 10.1016/j.mtphys.2023.101250
[10]Nielsen DU, Hu XM, Daasbjerg K, et al. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nature Catalysis. 2018, 1(4): 244-254. doi: 10.1038/s41929-018-0051-3
[11]Xu F, Wu D, Wang Z, et al. Synergistic effect and high performance of transition metal-anchored boron-doped graphyne electrocatalyst applied in the electroreduction of CO2 to C1 products: A DFT study. Applied Surface Science. 2023, 631: 157505. doi: 10.1016/j.apsusc.2023.157505
[12]Peterson AA, Abild-Pedersen F, Studt F, et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy & Environmental Science. 2010, 3(9): 1311. doi: 10.1039/c0ee00071j
[13]Oloman C, Li H. Electrochemical Processing of Carbon Dioxide. ChemSusChem. 2008, 1(5): 385-391. doi: 10.1002/cssc.200800015
[14]Hori Y. Electrochemical CO2 Reduction on Metal Electrodes. In: Vayenas CG, White RE, Gamboa-Aldeco ME (editors). Modern Aspects of Electrochemistry. Springer; 2008. pp. 89-189.
[15]Wei L, Meng D, Jiang Q, et al. A review on oxygen evolution electrocatalysts based on the different Ni-Fe matrix composites. Journal of Environmental Chemical Engineering. 2022, 10(6): 108591. doi: 10.1016/j.jece.2022.108591
[16]Pan F, Li B, Sarnello E, et al. Pore-Edge Tailoring of Single-Atom Iron–Nitrogen Sites on Graphene for Enhanced CO2 Reduction. ACS Catalysis. 2020, 10(19): 10803-10811. doi: 10.1021/acscatal.0c02499
[17]Su J, Pan D, Dong Y, et al. Ultrafine Fe2C Iron Carbide Nanoclusters Trapped in Topological Carbon Defects for Efficient Electroreduction of Carbon Dioxide. Advanced Energy Materials. 2023, 13(20). doi: 10.1002/aenm.202204391
[18]He J, Hu B, Zhao Y. Superaerophobic Electrode with Metal@Metal‐Oxide Powder Catalyst for Oxygen Evolution Reaction. Advanced Functional Materials. 2016, 26(33): 5998-6004. doi: 10.1002/adfm.201602116
[19]Wei X, Wei S, Cao S, et al. Cu acting as Fe activity promoter in dual-atom Cu/Fe-NC catalyst in CO2RR to C1 products. Applied Surface Science. 2021, 564: 150423. doi: 10.1016/j.apsusc.2021.150423
[20]Bagger A, Ju W, Varela AS, et al. Electrochemical CO2 Reduction: Classifying Cu Facets. ACS Catalysis. 2019, 9(9): 7894-7899. doi: 10.1021/acscatal.9b01899
[21]Cometto C, Ugolotti A, Grazietti E, et al. Copper single-atoms embedded in 2D graphitic carbon nitride for the CO2 reduction. npj 2D Materials and Applications. 2021, 5(1). doi: 10.1038/s41699-021-00243-y
[22]Kong Q, An X, Liu Q, et al. Copper-based catalysts for the electrochemical reduction of carbon dioxide: progress and future prospects. Materials Horizons. 2023, 10(3): 698-721. doi: 10.1039/d2mh01218a
[23]Yu P, Lv X, Wang Q, et al. Promoting Electrocatalytic CO2 Reduction to CH4 by Copper Porphyrin with Donor–Acceptor Structures. Small. 2022, 19(4). doi: 10.1002/smll.202205730
[24]Yang YL, Wang YR, Gao GK, et al. Self-assembly of single metal sites embedded covalent organic frameworks into multi-dimensional nanostructures for efficient CO2 electroreduction. Chinese Chemical Letters. 2022, 33(3): 1439-1444. doi: 10.1016/j.cclet.2021.08.063
[25]Liu B, Peng H, Cheng J, et al. Nitrogen‐Doped Graphene‐Encapsulated Nickel–Copper Alloy Nanoflower for Highly Efficient Electrochemical Hydrogen Evolution Reaction. Small. 2019, 15(48). doi: 10.1002/smll.201901545
[26]Portha JF, Parkhomenko K, Kobl K, et al. Kinetics of Methanol Synthesis from Carbon Dioxide Hydrogenation over Copper–Zinc Oxide Catalysts. Industrial & Engineering Chemistry Research. 2017, 56(45): 13133-13145. doi: 10.1021/acs.iecr.7b01323
[27]Ma Y, Wang J, Yu J, et al. Surface modification of metal materials for high-performance electrocatalytic carbon dioxide reduction. Matter. 2021, 4(3): 888-926. doi: 10.1016/j.matt.2021.01.007
[28]Bui TS, Lovell EC, Daiyan R, et al. Defective Metal Oxides: Lessons from CO2RR and Applications in NOXRR. Advanced Materials. 2023, 35(28). doi: 10.1002/adma.202205814
[29]De Luna P, Quintero-Bermudez R, Dinh CT, et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nature Catalysis. 2018, 1(2): 103-110. doi: 10.1038/s41929-017-0018-9
[30]Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nature Reviews Materials. 2017, 2(2). doi: 10.1038/natrevmats.2016.98
[31]Han M, Maleski K, Shuck CE, et al. Tailoring Electronic and Optical Properties of MXenes through Forming Solid Solutions. Journal of the American Chemical Society. 2020, 142(45): 19110-19118. doi: 10.1021/jacs.0c07395
[32]Xiao Y, Zhang W. High throughput screening of M3C2 MXenes for efficient CO2 reduction conversion into hydrocarbon fuels. Nanoscale. 2020, 12(14): 7660-7673. doi: 10.1039/c9nr10598k
[33]Li N, Chen X, Ong WJ, et al. Understanding of Electrochemical Mechanisms for CO2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes). ACS Nano. 2017, 11(11): 10825-10833. doi: 10.1021/acsnano.7b03738
[34]Otgonbayar Z, Oh WC. MXene-based nanocomposite for the photocatalytic CO2 reduction: Comprehensive review. Molecular Catalysis. 2023, 541: 113085. doi: 10.1016/j.mcat.2023.113085
[35]Sreedhar A, Reddy IN, Noh JS. Photocatalytic and electrocatalytic reduction of CO2 and N2 by Ti3C2 MXene supported composites for a cleaner environment: A review. Journal of Cleaner Production. 2021, 328: 129647. doi: 10.1016/j.jclepro.2021.129647
[36]Wang H, Li J, Li K, et al. Transition metal nitrides for electrochemical energy applications. Chemical Society Reviews. 2021, 50(2): 1354-1390. doi: 10.1039/d0cs00415d
[37]Hu H, Wang X, Attfield JP, et al. Metal nitrides for seawater electrolysis. Chemical Society Reviews. 2024, 53(1): 163-203. doi: 10.1039/d3cs00717k
[38]Gao B, Li X, Ding K, et al. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. Journal of Materials Chemistry A. 2019, 7(1): 14-37. doi: 10.1039/c8ta05760e
[39]Shan J, Sun K, Li H, et al. Composition regulation and defects introduction via amorphous CuEu alloy shell for efficient CO2 electroreduction toward methane. Journal of CO2 Utilization. 2020, 41: 101285. doi: 10.1016/j.jcou.2020.101285
[40]Yang B, Zeng J, Zhang Z, et al. Kinetic-boosted CO2 electroreduction to formate via synergistic electric-thermal field on hierarchical bismuth with amorphous layer. Journal of Energy Chemistry. 2024, 90: 233-243. doi: 10.1016/j.jechem.2023.11.022
[41]Cao Y, Zheng D, Zhang F, et al. Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: A review. Journal of Materials Science & Technology. 2022, 102: 232-263. doi: 10.1016/j.jmst.2021.05.078
[42]Zhang J, Liu J, Xi L, et al. Single-Atom Au/NiFe Layered Double Hydroxide Electrocatalyst: Probing the Origin of Activity for Oxygen Evolution Reaction. Journal of the American Chemical Society. 2018, 140(11): 3876-3879. doi: 10.1021/jacs.8b00752
[43]Zhang L, Peng J, Yuan Y, et al. Bifunctional heterostructure NiCo-layered double hydroxide nanosheets/NiCoP nanotubes/Ni foam for overall water splitting. Applied Surface Science. 2021, 557: 149831. doi: 10.1016/j.apsusc.2021.149831
[44]Iwase K, Hirano T, Honma I. Copper Aluminum Layered Double Hydroxides with Different Compositions and Morphologies as Electrocatalysts for the Carbon Dioxide Reduction Reaction. ChemSusChem. 2021, 15(2). doi: 10.1002/cssc.202102340
[45]Ma X, Liu T, Liu E, et al. Preparation and performance of Cd-MgAl-LDHs@RGO in high efficiency electrocatalytic reduction of CO2 to CO. Molecular Catalysis. 2023, 535: 112876. doi: 10.1016/j.mcat.2022.112876
[46]Zhang ZY, Tian H, Bian L, et al. Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products. Journal of Energy Chemistry. 2023, 83: 90-97. doi: 10.1016/j.jechem.2023.04.034
[47]He J, Dou T, Diao S, et al. Cu/Fe3O4 Nanocomposites from Layered Double Hydroxides as Catalysts for Selective Electroreduction of Carbon Dioxide. ACS Applied Nano Materials. 2023, 6(14): 13543-13550. doi: 10.1021/acsanm.3c02193
[48]Ma Y, Chen F, Yang Q, et al. Sulfate radical induced degradation of Methyl Violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate. Journal of Environmental Management. 2018, 227: 406-414. doi: 10.1016/j.jenvman.2018.08.030
[49]Anantharaj S, Noda S. Amorphous Catalysts and Electrochemical Water Splitting: An Untold Story of Harmony. Small. 2019, 16(2). doi: 10.1002/smll.201905779
[50]Tan BJ, Klabunde KJ, Sherwood PMA. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chemistry of Materials. 1990, 2(2): 186-191. doi: 10.1021/cm00008a021
[51]Wang WJ, Qiao MH, Yang J, et al. Selective hydrogenation of cyclopentadiene to cyclopentene over an amorphous NiB/SiO2 catalyst. Applied Catalysis A: General. 1997, 163(1): 101-109. doi: 10.1016/S0926-860X(97)00125-7
[52]Li T, Wang J, Zhu S, et al. Cu3PdxN nanocrystals for efficient CO2 electrochemical reduction to methane. Electrochimica Acta. 2021, 371: 137793. doi: 10.1016/j.electacta.2021.137793
[53]Butt FA, Alshahrani T, Awan ZUH, et al. Electrochemical CO2 reduction to gaseous methane and carbon monoxide using plasma-synthesized copper nanowires. Journal of Solid State Electrochemistry. 2023. doi: 10.1007/s10008-023-05600-z
[54]Roy S, Li Z, Chen Z, et al. Cooperative Copper Single‐Atom Catalyst in 2D Carbon Nitride for Enhanced CO2 Electrolysis to Methane. Advanced Materials. 2024. doi: 10.1002/adma.202300713
[55]Shang H, Kim D, Wallentine SK, et al. Ensemble effects in Cu/Au ultrasmall nanoparticles control the branching point for C1 selectivity during CO2 electroreduction. Chemical Science. 2021. doi: 10.1039/d1sc02602j
[56]Zhang T, Verma S, Kim S, et al. Highly dispersed, single-site copper catalysts for the electroreduction of CO2 to methane. Journal of Electroanalytical Chemistry. 2020, 875: 113862. doi: 10.1016/j.jelechem.2020.113862
[57]Wang Q, Wang X, Wu C, et al. Electrodeposition of tin on Nafion-bonded carbon black as an active catalyst layer for efficient electroreduction of CO2 to formic acid. Scientific Reports. 2017, 7(1). doi: 10.1038/s41598-017-14233-y
[58]Ahn ST, Sen S, Palmore GTR. Grazing incidence X-Ray diffraction: identifying the dominant facet in copper foams that electrocatalyze the reduction of carbon dioxide to formate. Nanoscale. 2022, 14(36): 13132-13140. doi: 10.1039/d2nr03212k
[59]Kattel S, Ramírez PJ, Chen JG, et al. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science. 2017, 355(6331): 1296-1299. doi: 10.1126/science.aal3573
[60]El-Nagar GA, Haun F, Gupta S, et al. Unintended cation crossover influences CO2 reduction selectivity in Cu-based zero-gap electrolysers. Nature Communications. 2023, 14(1). doi: 10.1038/s41467-023-37520-x
Copyright (c) 2024 Dian Song, Jinqing He, Yiping Wang, Xuhui Zhao, Fazhi Zhang, Xiaodong Lei

This work is licensed under a Creative Commons Attribution 4.0 International License.