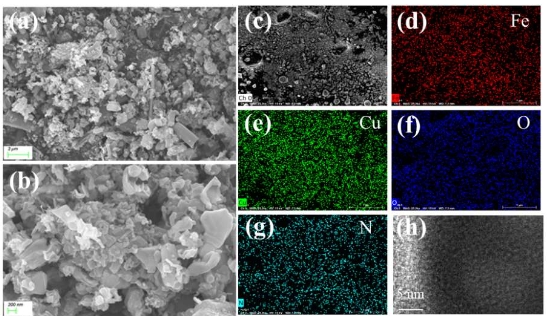
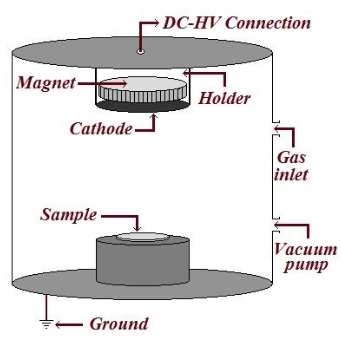
Research progress on hydroxide fluoride-based electrode materials for supercapacitors
Abstract
Supercapacitors have attracted much attention due to their high-power density and long cycle life, making them a potential substitute for traditional batteries. Research on hydroxide fluorides as electrode materials for supercapacitors has been increasing. Hydroxide fluorides exhibit higher specific capacitance due to the redox reactions between transition metal elements in different oxidation states. However, their high resistance limits their rate performance and cycling stability, which hinders their large-scale application. This article summarizes the main synthesis methods of hydroxide fluorides, and by controlling the reaction conditions, hydroxide fluorides with different morphologies and structures can be obtained to meet various application requirements. In addition, considering the limitations of hydroxide fluorides, this article systematically introduces the main approaches to improving their electrode performance and summarizes the electrochemical characteristics and latest research progress of hydroxide fluorides.
References
[1]Shi W, Meng Z, Xu Z, et al. Controllable vacancy strategy mediated by organic ligands of nickel fluoride alkoxides for high-performance aqueous energy storage. Journal of Materials Chemistry A. 2023, 11(3): 1369-1379. doi: 10.1039/d2ta08004d
[2]Meng Z, Gong X, Xu J, et al. A general strategy for preparing hollow spherical multilayer structures of Oxygen-Rich vacancy transition metal Oxides, especially high entropy perovskite oxides. Chemical Engineering Journal. 2023, 457: 141242. doi: 10.1016/j.cej.2022.141242
[3]Wang F, Wu X, Yuan X, et al. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chemical Society Reviews. 2017, 46(22): 6816-6854. doi: 10.1039/c7cs00205j
[4]Wang Y, Du Z, Xu J, et al. Improved Catalytic Activity and Stability of Co9S8 by Se Incorporation for Efficient Oxygen Evolution Reaction. Inorganic Chemistry. 2022, 61(51): 21139-21147. doi: 10.1021/acs.inorgchem.2c03805
[5]Sun X, Meng Z, Hao Z, et al. Efficient fabrication of flower-like core–shell nanochip arrays of lanthanum manganate and nickel cobaltate for high-performance supercapacitors. Journal of Colloid and Interface Science. 2023, 630: 618-628. doi: 10.1016/j.jcis.2022.10.035
[6]Olabi AG, Abbas Q, Al Makky A, et al. Supercapacitors as next generation energy storage devices: Properties and applications. Energy. 2022, 248: 123617. doi: 10.1016/j.energy.2022.123617
[7]Salanne M, Rotenberg B, Naoi K, et al. Efficient storage mechanisms for building better supercapacitors. Nature Energy. 2016, 1(6). doi: 10.1038/nenergy.2016.70
[8]Simon P, Gogotsi Y. Materials for electrochemical capacitors. Nature Materials. 2008, 7(11): 845-854. doi: 10.1038/nmat2297
[9]Aricò AS, Bruce P, Scrosati B, et al. Nanostructured materials for advanced energy conversion and storage devices. Nature Materials. 2005, 4(5): 366-377. doi: 10.1038/nmat1368
[10]Wang G, Zhang L, Zhang J. A review of electrode materials for electrochemical supercapacitors. Chemical Society Reviews. 2012, 41(2): 797-828. doi: 10.1039/c1cs15060j
[11]Chhowalla M, Shin HS, Eda G, et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nature Chemistry. 2013, 5(4): 263-275. doi: 10.1038/nchem.1589
[12]Zhang LL, Zhao XS. Carbon-based materials as supercapacitor electrodes. Chemical Society Reviews. 2009, 38(9): 2520. doi: 10.1039/b813846j
[13]Zhu Y, Murali S, Stoller MD, et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science. 2011, 332(6037): 1537-1541. doi: 10.1126/science.1200770
[14]Kötz R, Carlen M. Principles and applications of electrochemical capacitors. Electrochimica Acta. 2000, 45(15-16): 2483-2498. doi: 10.1016/s0013-4686(00)00354-6
[15]Frackowiak E, Béguin F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon. 2001, 39(6): 937-950. doi: 10.1016/s0008-6223(00)00183-4
[16]Chen LF, Zhang XD, Liang HW, et al. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano. 2012, 6(8): 7092-7102. doi: 10.1021/nn302147s
[17]Augustyn V, Simon P, Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy & Environmental Science. 2014, 7(5): 1597. doi: 10.1039/c3ee44164d
[18]Pandolfo AG, Hollenkamp AF. Carbon properties and their role in supercapacitors. Journal of Power Sources. 2006, 157(1): 11-27. doi: 10.1016/j.jpowsour.2006.02.065
[19]Snook GA, Kao P, Best AS. Conducting-polymer-based supercapacitor devices and electrodes. Journal of Power Sources. 2011, 196(1): 1-12. doi: 10.1016/j.jpowsour.2010.06.084
[20]Bonaccorso F, Colombo L, Yu G, et al. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science. 2015, 347(6217). doi: 10.1126/science.1246501
[21]Liu C, Yu Z, Neff D, et al. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Letters. 2010, 10(12): 4863-4868. doi: 10.1021/nl102661q
[22]Zhai Y, Dou Y, Zhao D, et al. Carbon Materials for Chemical Capacitive Energy Storage. Advanced Materials. 2011, 23(42): 4828-4850. doi: 10.1002/adma.201100984
[23]Chen H, Cong TN, Yang W, et al. Progress in electrical energy storage system: A critical review. Progress in Natural Science. 2009, 19(3): 291-312. doi: 10.1016/j.pnsc.2008.07.014
[24]Zhong C, Deng Y, Hu W, et al. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chemical Society Reviews. 2015, 44(21): 7484-7539. doi: 10.1039/c5cs00303b
[25]Luo X, Wang J, Dooner M, et al. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Applied Energy. 2015, 137: 511-536. doi: 10.1016/j.apenergy.2014.09.081
[26]Wang Y, Shi Z, Huang Y, et al. Supercapacitor Devices Based on Graphene Materials. The Journal of Physical Chemistry C. 2009, 113(30): 13103-13107. doi: 10.1021/jp902214f
[27]Wei W, Cui X, Chen W, et al. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chemical Society Reviews. 2011, 40(3): 1697-1721. doi: 10.1039/c0cs00127a
[28]Wan F, Wang X, Tang C, et al. Metallic 1T-MoS2 coupled with MXene towards ultra-high rate-capabilities for supercapacitors. Journal of Materials Chemistry A. 2022, 10(22): 12258-12268. doi: 10.1039/d2ta01908f
[29]Yu J, Xie F, Wu Z, et al. Flexible metallic fabric supercapacitor based on graphene/polyaniline composites. Electrochimica Acta. 2018, 259: 968-974. doi: 10.1016/j.electacta.2017.11.008
[30]Yu Z, Tetard L, Zhai L, et al. Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy & Environmental Science. 2015, 8(3): 702-730. doi: 10.1039/c4ee03229b
[31]Ling Z, Ren CE, Zhao MQ, et al. Flexible and conductive MXene films and nanocomposites with high capacitance. Proceedings of the National Academy of Sciences. 2014, 111(47): 16676-16681. doi: 10.1073/pnas.1414215111
[32]Zhang K, Zhang LL, Zhao XS, et al. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chemistry of Materials. 2010, 22(4): 1392-1401. doi: 10.1021/cm902876u
[33]González A, Goikolea E, Barrena JA, et al. Review on supercapacitors: Technologies and materials. Renewable and Sustainable Energy Reviews. 2016, 58: 1189-1206. doi: 10.1016/j.rser.2015.12.249
[34]Yan J, Wang Q, Wei T, et al. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Advanced Energy Materials. 2013, 4(4). doi: 10.1002/aenm.201300816
[35]Wang H, Casalongue HS, Liang Y, et al. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. Journal of the American Chemical Society. 2010, 132(21): 7472-7477. doi: 10.1021/ja102267j
[36]Yan J, Fan Z, Sun W, et al. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Advanced Functional Materials. 2012, 22(12): 2632-2641. doi: 10.1002/adfm.201102839
[37]Fan Z, Yan J, Wei T, et al. Asymmetric Supercapacitors Based on Graphene/MnO2 and Activated Carbon Nanofiber Electrodes with High Power and Energy Density. Advanced Functional Materials. 2011, 21(12): 2366-2375. doi: 10.1002/adfm.201100058
[38]Pershaanaa M, Bashir S, Ramesh S, et al. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. Journal of Energy Storage. 2022, 50: 104599. doi: 10.1016/j.est.2022.104599
[39]Zhang J, Gong X, Li X, et al. Electron-ion conjugation sites co-constructed by defects and heteroatoms assisted carbon electrodes for high-performance aqueous energy storage. Journal of Colloid and Interface Science. 2023, 640: 600-609. doi: 10.1016/j.jcis.2023.02.147
[40]Zeng F, Meng Z, Xu Z, et al. Biomass-derived porous activated carbon for ultra-high performance supercapacitor applications and high flux removal of pollutants from water. Ceramics International. 2023, 49(10): 15377-15386. doi: 10.1016/j.ceramint.2023.01.122
[41]Zhi M, Xiang C, Li J, et al. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale. 2013, 5(1): 72-88. doi: 10.1039/c2nr32040a
[42]Futaba DN, Hata K, Yamada T, et al. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nature Materials. 2006, 5(12): 987-994. doi: 10.1038/nmat1782
[43]Lin T, Chen IW, Liu F, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science. 2015, 350(6267): 1508-1513. doi: 10.1126/science.aab3798
[44]Frackowiak E. Carbon materials for supercapacitor application. Physical Chemistry Chemical Physics. 2007, 9(15): 1774. doi: 10.1039/b618139m
[45]Zhu B, Liu Y, Zhao H, et al. ZnOHF/N-doped carbon hybrids as a novel anode material for enhanced lithium storage. Journal of Alloys and Compounds. 2021, 889: 161705. doi: 10.1016/j.jallcom.2021.161705
[46]Chen H, Zhang Y, Yang J, et al. Ni0.33Co0.66(OH)F hollow hexagons woven by MWCNTs for high-performance lithium-ion batteries. Journal of Materials Chemistry A. 2015, 3(41): 20690-20697. doi: 10.1039/c5ta05143f
[47]Wu F, Ma X, Feng J, et al. 3D Co3O4and CoO@C wall arrays: morphology control, formation mechanism, and lithium-storage properties. Journal of Materials Chemistry A. 2014, 2(30): 11597. doi: 10.1039/c4ta01676a
[48]Ni S, Ma J, Zhang J, et al. Facile synthesis of Co(OH)F micro-rods and its application as anode for lithium ion batteries. Materials Letters. 2015, 139: 138-140. doi: 10.1016/j.matlet.2014.10.035
[49]Jiang S, Pang M, Zhao J, et al. Superior performance asymmetric supercapacitors based on a directly grown three-dimensional lawn-like cobalt-zinc hydroxyfluorides nanoneedle arrays electrode. Chemical Engineering Journal. 2017, 326: 1048-1057. doi: 10.1016/j.cej.2017.06.017
[50]Li X, Ding R, Shi W, et al. Hierarchical porous Co(OH)F/Ni(OH)2: A new hybrid for supercapacitors. Electrochimica Acta. 2018, 265: 455-473. doi: 10.1016/j.electacta.2018.01.194
[51]Zhang JF, Wang Y, Shu X, et al. One-pot synthesis of nickel-cobalt hydroxyfluorides nanowires with ultrahigh energy density for an asymmetric supercapacitor. Science Bulletin. 2018, 63(5): 322-330. doi: 10.1016/j.scib.2018.01.024
[52]Chen S, Zhou X, Ma X, et al. Asymmetric supercapacitors with excellent rate performance by integrating Co(OH)F nanorods and layered Ti3C2Tx paper. RSC Advances. 2019, 9(53): 30957-30963. doi: 10.1039/c9ra06393e
[53]Dong Q, Su T, Ge W, et al. Atomic Doping and Anion Reconstructed CoF2 Electrocatalyst for Oxygen Evolution Reaction. Advanced Materials Interfaces. 2020, 7(7). doi: 10.1002/admi.201901939
[54]Zhang B, Jiang K, Wang H, et al. Fluoride-Induced Dynamic Surface Self-Reconstruction Produces Unexpectedly Efficient Oxygen-Evolution Catalyst. Nano Letters. 2018, 19(1): 530-537. doi: 10.1021/acs.nanolett.8b04466
[55]Roger I, Shipman MA, Symes MD. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nature Reviews Chemistry. 2017, 1(1). doi: 10.1038/s41570-016-0003
[56]Zhang L, Wang W, Xu G, et al. Facile synthesis of CoxFe1−xP microcubes derived from metal-organic frameworks for efficient oxygen evolution reaction. Journal of Colloid and Interface Science. 2019, 554: 202-209. doi: 10.1016/j.jcis.2019.07.008
[57]Shi W, Jiang C, Meng Z, et al. Novel Co-doped nickel hydroxyfluorides with rapid electron transfer for high-performance supercapacitors. Journal of Alloys and Compounds. 2023, 959: 170558. doi: 10.1016/j.jallcom.2023.170558
[58]Xu F, Sun L, Dai M, et al. Fluorine-Ion-Mediated Electrodeposition of Rhombus-Like ZnFOH Nanorod Arrays: An Intermediate Route to Novel ZnO Nanoarchitectures. The Journal of Physical Chemistry C. 2010, 114(36): 15377-15382. doi: 10.1021/jp1066082
[59]Zhu L, Zheng Y, Hao T, et al. Synthesis of hierarchical ZnO nanobelts via Zn(OH)F intermediate using ionic liquid-assistant microwave irradiation method. Materials Letters. 2009, 63(28): 2405-2408. doi: 10.1016/j.matlet.2009.07.062
[60]Song J kui, Zheng M bo, Yang Z jiang, et al. Synthesis of Novel Flower-Like Zn(OH)F via a Microwave-Assisted Ionic Liquid Route and Transformation into Nanoporous ZnO by Heat Treatment. Nanoscale Research Letters. 2009, 4(12). doi: 10.1007/s11671-009-9428-1
[61]Barzegar F, Bello A, Momodu DY, et al. Effect of radiation on the performance of activated carbon base supercapacitor: Part I. Influence of microwave irradiation exposure on electrodes material. Energy Procedia. 2019, 158: 4554-4559. doi: 10.1016/j.egypro.2019.01.754
[62]Peng Y, Zhou HY, Wang ZH. Synthesis, characterization and photocatalytic activity of Zn(OH)F hierarchical nanofibers prepared by a simple solution-based method. CrystEngComm. 2012, 14(8): 2812. doi: 10.1039/c2ce06389a
[63]Ahmad S, Rawat P, Nagarajan R. Facile green synthesis of Zn(OH)F from the single source precursor KZnF3. Materials Letters. 2015, 139: 86-88. doi: 10.1016/j.matlet.2014.10.037
[64]Lemoine K, Zhang L, Dambournet D, et al. Synthesis by Thermal Decomposition of Two Iron Hydroxyfluorides: Structural Effects of Li Insertion. Chemistry of Materials. 2019, 31(11): 4246-4257. doi: 10.1021/acs.chemmater.9b01252
[65]Lv J, Yang X, Zang HY, et al. Ultralong needle-like N-doped Co(OH)F on carbon fiber paper with abundant oxygen vacancies as an efficient oxygen evolution reaction catalyst. Materials Chemistry Frontiers. 2018, 2(11): 2045-2053. doi: 10.1039/c8qm00405f
[66]Hao Z, Jiang C, Xu Z, et al. Reasonably optimized structure of iron-doped cobalt hydroxylfluoride for high-performance supercapacitors. Journal of Colloid and Interface Science. 2023, 644: 64-72. doi: 10.1016/j.jcis.2023.04.061
[67]Chen S, Song Y, Zhou X, et al. Co(OH)F nanorods@KxMnO2 nanosheet core–shell structured arrays for pseudocapacitor application. RSC Advances. 2019, 9(62): 36208-36212. doi: 10.1039/c9ra07024a
Copyright (c) 2023 Zijin Xu, Zeshuo Meng

This work is licensed under a Creative Commons Attribution 4.0 International License.