New Breakthrough in CO2 Electroreduction: Nitrided Copper-Iron Composite Oxides Transform Carbon Dioxide into Valuable Chemicals!
We are excited to announce a groundbreaking study published in the latest issue of Energy Storage and Conversion (Volume 2, Issue 2, 2024), featuring a novel material that significantly advances the field of carbon dioxide (CO2) electroreduction. The article introduces a new class of catalysts derived from layered double hydroxides (LDHs) that have demonstrated exceptional performance in converting CO2 into methane (CH4) and formic acid (HCOOH).
Revolutionizing CO2 Reduction with Nitrided Copper-Iron Oxides
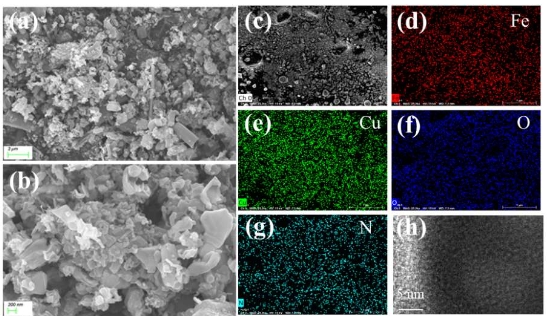
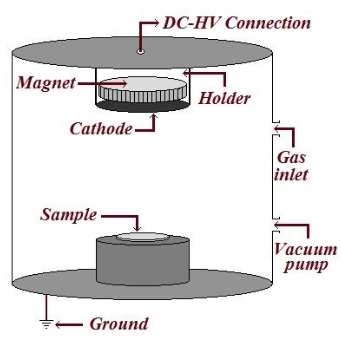
Researchers at the State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, have developed an innovative approach to electrocatalytic CO2 reduction. By utilizing a gas-phase nitriding process with urea as the nitrogen source, they have successfully synthesized amorphous nitrided copper-iron oxides from CuFe-LDH precursors. This material has shown a remarkable total Faraday efficiency of 74.7% at -0.7 V vs. RHE, coupled with impressive durability in H-cell testing for continuous 10-hour periods.
Addressing Global Warming with Advanced Material Science
The study addresses the pressing issue of global warming by targeting the major contributor to this crisis—CO2 emissions. The combustion of fossil fuels is a significant source of atmospheric CO2, and this research offers a promising solution to transform these emissions into valuable chemical products, thereby contributing to a more sustainable future.
In-Depth Analysis and Cutting-Edge Techniques
The research team conducted a comprehensive analysis of the synthesized material using state-of-the-art techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and X-ray photoelectron spectroscopy (XPS). These analyses revealed the amorphous nature of the material, which is crucial for generating active defect sites that enhance catalytic reactions.
Electrochemical Performance and Reaction Mechanisms
The electrochemical performance of the nitrided copper-iron composite oxides was evaluated in a 0.5 M KHCO3 electrolyte, demonstrating a lower onset potential and high selectivity for C1 products. The team also employed in situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) to elucidate the reaction mechanisms, providing insights into the conversion of CO2 to *COOH and subsequent formation of HCOOH and CH4.
A Promising Future for CO2 Electroreduction Catalysts
This study not only showcases the potential of amorphous copper-iron oxide nitrides as catalysts for CO2 electroreduction but also paves the way for the design of more efficient catalysts. The findings are a testament to the commitment of the scientific community to tackle environmental challenges through advanced material science and electrochemistry.
Acknowledgments and Contributions
The article acknowledges the contributions of all authors and the support from the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the Program for Changjiang Scholars and Innovative Research Teams in University.
For more detailed information, tables, and figures, please refer to the article content.
About Energy Storage and Conversion: Energy Storage and Conversion is a leading journal dedicated to publishing high-quality research on energy-related topics, fostering innovation, and promoting sustainable energy solutions.
Join us in celebrating this significant advancement in the quest for sustainable energy and environmental solutions.