Manganese and iron-doped yttrium borate as an excellent multifunctional inorganic material
Abstract
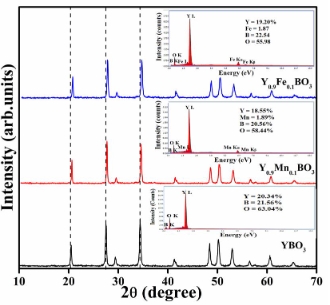
Manganese and iron-doped π-YBO3 have been synthesized using a modified epoxide-mediated gel method. The PXRD pattern evaluated the formation of the desired phase and the structural changes. EDS spectra determined the elemental analysis of undoped and doped samples. Raman spectra observed the stretching and bending modes of B-O bonds. The direct band gaps for doped samples were 1.47 and 2.07 eV, respectively, lower than the band gap value of 5.81 eV for π-YBO3. The green and blue indigo emission bands were observed in the photoluminescence spectra. Doped samples showed good magnetic properties as they are antiferromagnetic and ferromagnetic at low temperature (T = 5 K) M-H plot and SQUID measurement. An indigenously built Sawyer-Tower circuit is used to measure ferroelectric hysteresis. Photodegradation studies of RhB were conducted under UV-visible irradiation.
References
[1]Rowsell JLC, Gaubicher J, Nazar LF. A new class of materials for lithium-ion batteries: Iron (III) borates. Journal of Power Sources 2001; 97–98: 254–257. doi: 10.1016/S0378-7753(01)00532-8
[2]Kurtzig AJ, Wolfe R, LeCraw RC, Nielsen JW. Magneto-optical properties of a green room-temperature ferromagnet: FeBO3. Applied Physics Letters 1969; 14(11): 350–352. doi: 10.1063/1.1652682
[3]Bither TA, Frederick CG, Gier TE, et al. Ferromagnetic VBO3 and antiferromagnetic CrBO3. Solid State Communications 1970; 8(2): 109–112. doi: 10.1016/0038-1098(70)90582-X
[4]Takéuchi Y, Watanabe T, Ito T. The crystal structures of warwickite, ludwigite and pinakiolite. Acta Crystallographica 1950; 3: 98–107. doi: 10.1107/S0365110X50000252
[5]Norrestam R, Kritikos M, Sjödin A. Manganese (II, III) oxyborate, Mn2OBO3: A distorted homometallic warwickite—Synthesis, crystal structure, band calculations, and magnetic susceptibility. Journal of Solid State Chemistry 1995; 114(2): 311–316. doi: 10.1006/jssc.1995.1049
[6]Attfield JP, Clarke JF, Perkins DA. Magnetic and crystal structures of iron borates. Physica B: Condensed Matter 1992; 180–181: 581–584. doi: 10.1016/0921-4526(92)90401-D
[7]Bertaut EF. Structures des boroferrites. Acta Crystallographica 1950; 3: 473–474. doi: 10.1107/S0365110X50001312
[8]Li HK, Wang L, Cai GM, et al. Synthesis and crystal structure of a novel ludwigite borate: Mg2InBO5. Journal of Alloys and Compounds 2013; 575: 104–108. doi: 10.1016/j.jallcom.2013.04.024
[9]Perkins DA, Attfield JP. Resonant powder X-ray determination of the cation distribution in FeNi2BO5. Journal of the Chemical Society, Chemical Communications 1991; 4: 229–231. doi: 10.1039/C39910000229
[10]Fuchs B, Schröder F, Heymann G, et al. Crystal structure re-determination, spectroscopy, and photoluminescence of π-YBO3: Eu3+. Journal of Inorganic and General Chemistry 2021; 647(22): 2035–2046. doi: 10.1002/zaac.202100229
[11]Pitscheider A, Kaindl R, Oeckler O, Huppertz H. The crystal structure of π-ErBO3: New single-crystal data for an old problem. Journal of Solid State Chemistry 2011; 184(1): 149–153. doi: 10.1016/j.jssc.2010.11.018
[12]Lin J, Sheptyakov D, Wang Y, Allenspach P. Structures and phase transition of vaterite-type rare earth orthoborates: A neutron diffraction study. Chemistry of Materials 2004; 16(12): 2418–2424. doi: 10.1021/cm0499388
[13]Morgan PED, Carroll PJ, Lange FF. Crystal structure of YSiO2N and a reappraisal of the “vaterite” type, YBO3. Materials Research Bulletin 1977; 12(3): 251–259. doi: 10.1016/0025-5408(77)90142-8
[14]Levin EM, Roth RS, Martin JB. Polymorphism of ABO3 type rare earth borates. American Mineralogist: Journal of Earth and Planetary Materials 1961; 46(9–10): 1030–1055.
[15]Newnham RE, Redman MJ, Santoro RP. Crystal structure of yttrium and other rare-earth borates. Journal of the American Ceramic Society 1963; 46(6): 253–256. doi: 10.1111/j.1151-2916.1963.tb11721.x
[16]Weir CE, Lippincott ER. Infrared studies of aragonite, calcite, and vaterite type structures in the borates, carbonates, and nitrates. Journal of Research of the National Bureau of Standards. Section A, Physics and Chemistry 1961; 65A(3): 173–180. doi: 10.6028/jres.065A.021
[17]Weir CE, Schroeder RA. Infrared spectra of the crystalline inorganic borates. Journal of Research of the National Bureau of Standards–A. Physics and Chemistry 1964; 68A(5): 465–487. doi: 10.6028/jres.068A.045
[18]Laperches JP, Tarte P. Infrared absorption spectra of rare earth borates (French). Spectrochimica Acta 1966; 22(7): 1201–1210. doi: 10.1016/0371-1951(66)80023-1
[19]Kriz HM, Bray PJ. On the crystal structure of YBO3, a vaterite-type borate. The Journal of Chemical Physics 1969; 51(8): 3624–3625. doi: 10.1063/1.1672566
[20]Denning JH, Boss SD. The vibrational spectra and structures of some rare-earth borates. Spectrochimica Acta Part A: Molecular Spectroscopy 1972; 28(9): 1775–1785. doi: 10.1016/0584-8539(72)80148-X
[21]Bradley WF, Graf DL, Roth RS. The vaterite-type ABO3 rare-earth borates. Acta Crystallographica 1966; 20: 283–287. doi: 10.1107/S0365110X66000549
[22]Chadeyron G, El-Ghozzi M, Mahiou R, Cousseins JC. Revised structure of the orthoborate YBO3. Journal of Solid State Chemistry 1997; 128(2): 261–266. doi: 10.1006/jssc.1996.7207
[23]Ren M, Lin JH, Dong Y, et al. Structure and phase transition of GdBO3. Chemistry of Materials 1999; 11(6): 1576–1580. doi: 10.1021/cm990022o
[24]Cohen-Adad MT, Aloui-Lebbou O, Goutaudier C, et al. Gadolinium and yttrium borates: Thermal behavior and structural considerations. Journal of Solid State Chemistry 2000; 154(1): 204–213. doi: 10.1006/jssc.2000.8837
[25]Hosokawa S, Tanaka Y, Iwamoto S, Inoue M. Morphology and structure of rare earth borate (REBO3) synthesized by glycothermal reaction. Journal of Materials Science 2008; 43: 2276–2285. doi: 10.1007/s10853-007-2023-x
[26]Mahato SS, Mahata D, Panda S, Mahata S. Perspective chapter: Sol-gel science and technology in context of nanomaterials—Recent advances. Sol-Gel Method-Recent Advances 2023. doi: 10.5772/intechopen.111378
[27]Gupta P, Sahni M, Chauhan S. Enhanced photoluminescence properties of rare earth elements doped Y0.50Gd0.50BO3 phosphor and its application in red and green LEDs. Optik 2021; 240: 166810. doi: 10.1016/j.ijleo.2021.166810
[28]Gupta P, Tomar R, Singh NB. Chromium-doped Y0.50Gd0.50BO3 as an excellent photocatalytic and magnetic material. Bulletin of Materials Science 2021; 44: 251. doi: 10.1007/s12034-021-02541-z
[29]Gu GX, Wang D, Lv XS, et al. In situ study on the structural transition in YBO3 through Raman spectroscopy. Materials Chemistry and Physics 2011; 131(1–2): 274–277. doi: 10.1016/j.matchemphys.2011.09.041
[30]Boonchom B, Baitahe R. Synthesis and characterization of nanocrystalline manganese pyrophosphate Mn2P2O7. Materials Letters 2009; 63(26): 2218–2220. doi: 10.1016/j.matlet.2009.07.028
[31]Orhan A, Aydin C, Aydin H, et al. Synthesis and optical properties of iron doped gallium nitride nanostructures by sol gel method. Microsystem Technologies 2015; 21: 1219–1224. doi: 10.1007/s00542-014-2273-x
[32]Lever ABP. Inorganic Electronic Spectroscopy, 2nd ed. Elsevier; 1984.
[33]You H, Zhang J, Hong G, Zhang H. Luminescent properties of Mn2+ in hexagonal aluminates under ultraviolet and vacuum ultraviolet excitation. The Journal of Physical Chemistry C 2007; 111(28): 10657–10661. doi: 10.1021/jp071889+
[34]Li RK, Greaves C. YCa3(MnO)3(BO3)4: A manganese borate containing ferromagnetic chains on a kagomé lattice. Physical Review B 2003; 68(17): 172403. doi: 10.1103/PhysRevB.68.172403
[35]Continentino MA, Pedreira AM, Guimaraes RB, et al. Specific heat and magnetization studies of Fe2OBO3, Mn2OBO3 and MgScOBO3. Physical Review B 2001; 64: 014406. doi: 10.1103/PhysRevB.64.014406
[36]Sultan K, Ikram M, Gautam S, et al. Electrical and magnetic properties of the pulsed laser deposited Ca doped LaMnO3 thin films on Si (100) and their electronic structures. RSC Advances 2015; 5(85): 69075–69085. doi: 10.1039/C5RA08028B
[37]Kumari K. Magnetic and dielectric properties of Fe3BO6 nanoplates prepared through self-combustion method. Journal of Advanced Dielectrics 2017; 7(6): 1750043. doi: 10.1142/S2010135X17500436
[38]Matteppanavar S, Angadi B, Rayaprol S. Low temperature magnetic studies on PbFe0.5Nb0.5O3 multiferroic. Physica B: Condensed Matter 2014; 448: 229–232. doi: 10.1016/j.physb.2014.04.024
[39]Rivas-Murias B, Rivadulla F, Sánchez-Andújar M, et al. Role of t2g versus eg interactions in the physical properties of A2OBO3 (A = Mn, Fe). Chemistry of Materials 2006; 18(19): 4547–4552. doi: 10.1021/cm0609698
[40]Maignan A, Lainé F, Guesdon A, et al. Charge ordering and multiferroicity in Fe3BO5 and Fe2MnBO5 oxyborates. Journal of Solid State Chemistry 2017; 246: 209–213. doi: 10.1016/j.jssc.2016.11.034
[41]Karmakar R, Neogi SK, Midya N, et al. Magnetic properties of Mn doped ZnO: The role of synthesis route. Journal of Materials Science: Materials in Electronics 2016; 27: 6371–6381. doi: 10.1007/s10854-016-4572-8
[42]Ke S, Lin P, Zeng X, et al. Tuning of dielectric and ferroelectric properties in single phase BiFeO3 ceramics with controlled Fe2+/Fe3+ ratio. Ceramics International 2014; 40(4): 5263–5268. doi: 10.1016/j.ceramint.2013.10.098
[43]Tomar R, Abdala AA, Chaudhary RG, et al. Photocatalytic degradation of dyes by nanomaterials. Materials Today: Proceedings 2020; 29: 967–973. doi: 10.1016/j.matpr.2020.04.144
[44]Zhong HE, Shaogui Y, Yongming JU, et al. Microwave photocatalytic degradation of rhodamine B using TiO2 supported on activated carbon: Mechanism implication. Journal of Environmental Sciences 2009; 21(2): 268–272. doi: 10.1016/S1001-0742(08)62262-7
[45]Su S, Guo W, Leng Y, et al. Heterogeneous activation of Oxone by CoxFe3−xO4 nanocatalysts for degradation of rhodamine B. Journal of Hazardous Materials 2013; 244–245: 736–742. doi: 10.1016/j.jhazmat.2012.11.005
[46]Yadav AA, Kang SW, Hunge YM. Photocatalytic degradation of rhodamine B using graphitic carbon nitride photocatalyst. Journal of Materials Science: Materials in Electronics 2021; 32: 15577–15585. doi: 10.1007/s10854-021-06106-y
[47]Hunge YM. Photoelectrocatalytic degradation of methylene blue using spray deposited ZnO thin films under UV illumination. MOJ Polymer Science 2017; 1(4): 135–139. doi: 10.15406/mojps.2017.01.00020
[48]Kale V, Hunge YM, Kamble SA, et al. Modification of energy level diagram of nano-crystalline ZnO by its composites with ZnWO4 suitable for sunlight assisted photo catalytic activity. Materials Today Communications 2021; 26: 102101. doi: 10.1016/j.mtcomm.2021.102101
[49]Pradeev Raj K, Sadaiyandi K, Kennedy A, Sagadevan S. Photocatalytic and antibacterial studies of indium-doped ZnO nanoparticles synthesized by co-precipitation technique. Journal of Materials Science: Materials in Electronics 2017; 28: 19025–19037. doi: 10.1007/s10854-017-7857-7
[50]Moulai F, Fellahi O, Messaoudi B, et al. Electrodeposition of nanostructured γ-MnO2 film for photodegradation of rhodamine B. Ionics 2018; 24: 2099–2109. doi: 10.1007/s11581-018-2440-7
Copyright (c) 2023 Pankaj Gupta, Mohit Sahni

This work is licensed under a Creative Commons Attribution 4.0 International License.