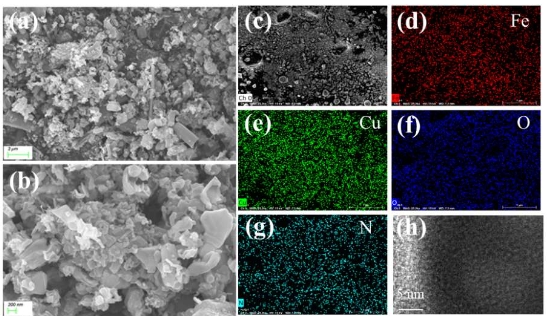
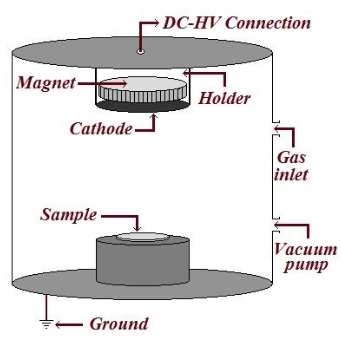
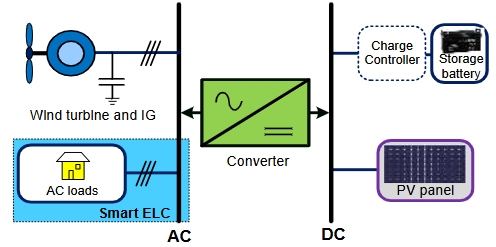
Dear colleagues,
Energy storage involves converting energy from forms that are difficult to store to more conveniently or economically storable forms. The energy storage technologies used include mechanical storage, thermal storage, electrochemical storage, other chemical storage (i.e. hydrogen storage, bio fuels), etc. In all systems, energy storage media are needed; for example, electrochemical energy storage can be achieved by converting chemical energy to electric energy and back, coupled with electron and ion transfer in electrode materials. Fortunately, solid materials with given chemical composition and crystal structure can store electrons and ions. More importantly, solid materials are easy to carry and use compared with liquid or gas materials when used in energy storage devices. Every breakthrough of electrochemical energy storage technologies is dependent on the progress of materials chemistry. This focuses, either on the chemistry used to produce the materials, or on the properties or applications of the materials produced, which creatively designs solid materials with various physical, chemical and electrochemical properties.
In order to achieve this goal, materials are constructed of multiple-scale functional units: for example, at the microscopic scale, different elements, electronic structures, crystal structures; at the mesoscopic scale different_ surface/interface structures; at the macroscopic scale, various morphologies, sizes, material_architectures (_hollow, core-shell, tube, wire-like). Studied methods for materials chemistry include experimental and theoretical routes, namely multinuclear and NMR studies of mechanisms, atomic structure, ion dynamics, electrochemical thermodynamics and kinetics, etc. In addition, novel interdisciplinary research methods have been introduced into the materials chemical field to accelerate the development of new materials for energy storage, such as materials genome initiative (MGI), nature-inspired materials, and atoms to product methods (A2P). Electrode materials with high energy density, high power density, low self-discharge, light, cheap, higher number of charge/discharge cycles, and high electronic and ionic conductivity determine the level of electric energy storage techniques. In this section, with the use of well-founded knowledge of materials chemistry, providing general rules to rationality design electrode materials with improved electrochemical performance, we will publish research articles/reviews supporting materials design toward applications in electrochemical energy storage, mainly including rechargeable batteries (lithium ion batteries, sodium ion batteries, aluminum-air batteries, etc.), flow batteries, transition-metal-based supercapacitors and supercapacitors employing redox active biomolecules as the faradic-type active material, etc., by either the chemistry used to produce the materials, (LiCoO2 , graphite, LiFePO4, activated carbon materials, RuO2, nature-inspired electrochemically active materials, etc.) on the properties of the materials produced.. Electrochemical Energy Storage Materials and Devices provides an opportunity for the interaction among chemists, physical-chemists, materials scientists, chemical engineers and industrialists interested in the scientific and technological aspects of advanced electrode materials for application in electrochemical energy storage. Seminal research articles and reviews in this field of study are welcome.
We look forward to receive your contributions.
Prof. Dr. César A.C. Sequeira
Section Editor