Applications of reinforcement learning, machine learning, and virtual screening in SARS-CoV-2-related proteins
Abstract
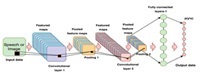
Similarly, to all coronaviruses, SARS-CoV-2 uses the S glycoprotein to enter host cells, which contains two functional domains: S1 and S2 receptor binding domain (RBD). Angiotensin-converting enzyme 2 (ACE2) is recognizable by the S proteins on the surface of the SARS-CoV-2 virus. The SARS-CoV-2 virus causes SARS, but some mutations in the RBD of the S protein markedly enhance their binding affinity to ACE2. Searching for new compounds in COVID-19 is an important initial step in drug discovery and materials design. Still, the problem is that this search requires trial-and-error experiments, which are costly and time-consuming. In the automatic molecular design method based on deep reinforcement learning, it is possible to design molecules with optimized physical properties by combining a newly devised coarse-grained representation of molecules with deep reinforcement learning. Also, structured-based virtual screening uses protein 3D structure information to evaluate the binding affinity between proteins and compounds based on physicochemical interactions such as van der Waals forces, Coulomb forces, and hydrogen bonds, and select drug candidate compounds. In addition, AlphaFold can predict 3D protein structures, given the amino acid sequence, and the protein building blocks. Ensemble docking, in which multiple protein structures are generated using the molecular dynamics method and docking calculations are performed for each, is often performed independently of docking calculations. In the future, the AlphaFold algorithm can be used to predict various protein structures related to COVID-19.
References
[1]Yashavantha Rao HC, Jayabaskaran C. The emergence of a novel coronavirus (SARS‐CoV‐2) disease and their neuroinvasive propensity may affect in COVID‐19 patients. Journal of Medical Virology. 2020; 92(7): 786-790. doi: 10.1002/jmv.25918
[2]Ma Y, Deng J, Liu Q, et al. Long-Term Consequences of Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(2): 1613. doi: 10.3390/ijerph20021613
[3]Bongiovanni M, De Lauretis A, Manes G, et al. Clinical characteristics and outcome of COVID-19 pneumonia in elderly subjects. Journal of Infection. 2021; 82(2): e33-e34. doi: 10.1016/j.jinf.2020.08.023
[4]Gupta SK, Minocha R, Thapa PJ, et al. Role of the Pangolin in Origin of SARS-CoV-2: An Evolutionary Perspective. International Journal of Molecular Sciences. 2022; 23(16): 9115. doi: 10.3390/ijms23169115
[5]Yaşar Ş, Çolak C, Yoloğlu S. Artificial Intelligence-Based Prediction of Covid-19 Severity on the Results of Protein Profiling. Computer Methods and Programs in Biomedicine. 2021; 202: 105996. doi: 10.1016/j.cmpb.2021.105996
[6]Dey L, Chakraborty S, Mukhopadhyay A. Machine learning techniques for sequence-based prediction of viral–host interactions between SARS-CoV-2 and human proteins. Biomedical Journal. 2020; 43(5): 438-450. doi: 10.1016/j.bj.2020.08.003
[7]Cihan P, Ozger ZB. A new approach for determining SARS-CoV-2 epitopes using machine learning-based in silico methods. Computational Biology and Chemistry. 2022; 98: 107688. doi: 10.1016/j.compbiolchem.2022.107688
[8]Alluwaimi AM, Alshubaith IH, Al-Ali AM, et al. The Coronaviruses of Animals and Birds: Their Zoonosis, Vaccines, and Models for SARS-CoV and SARS-CoV2. Frontiers in Veterinary Science. 2020; 7. doi: 10.3389/fvets.2020.582287
[9]Kesheh MM, Hosseini P, Soltani S, et al. An overview on the seven pathogenic human coronaviruses. Reviews in Medical Virology. 2021; 32(2). doi: 10.1002/rmv.2282
[10]Alexandersen S, Chamings A, Bhatta TR. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nature Communications. 2020; 11(1). doi: 10.1038/s41467-020-19883-7
[11]Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2020; 1866(10): 165878. doi: 10.1016/j.bbadis.2020.165878
[12]Chateau A, Van der Verren SE, Remaut H, et al. The Bacillus anthracis Cell Envelope: Composition, Physiological Role, and Clinical Relevance. Microorganisms. 2020; 8(12): 1864. doi: 10.3390/microorganisms8121864
[13]Bai C, Zhong Q, Gao GF. Overview of SARS-CoV-2 genome-encoded proteins. Science China Life Sciences. 2021; 65(2): 280-294. doi: 10.1007/s11427-021-1964-4
[14]Dërmaku-Sopjani M, Sopjani M. Interactions between ACE2 and SARS-CoV-2 S Protein: Peptide Inhibitors for Potential Drug Developments Against COVID-19. Current Protein & Peptide Science. 2021; 22(10): 729-744. doi: 10.2174/1389203722666210916141924
[15]Wartecki A, Rzymski P. On the Coronaviruses and Their Associations with the Aquatic Environment and Wastewater. Water. 2020; 12(6): 1598. doi: 10.3390/w12061598
[16]Roy AN, Gupta AM, Banerjee D, et al. Unraveling DPP4 Receptor Interactions with SARS-CoV-2 Variants and MERS-CoV: Insights into Pulmonary Disorders via Immunoinformatics and Molecular Dynamics. Viruses. 2023; 15(10): 2056. doi: 10.3390/v15102056
[17]Scialo F, Daniele A, Amato F, et al. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020; 198(6): 867-877. doi: 10.1007/s00408-020-00408-4
[18]Shirbhate E, Pandey J, Patel VK, et al. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacological Reports. 2021; 73(6): 1539-1550. doi: 10.1007/s43440-021-00303-6
[19]Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581(7807): 215-220. doi: 10.1038/s41586-020-2180-5
[20]Huang Y, Yang C, Xu X feng, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica. 2020; 41(9): 1141-1149. doi: 10.1038/s41401-020-0485-4
[21]Li X, Yuan H, Li X, et al. Spike protein mediated membrane fusion during SARS‐CoV‐2 infection. Journal of Medical Virology. 2022; 95(1). doi: 10.1002/jmv.28212
[22]Raghuvamsi PV, Tulsian NK, Samsudin F, et al. SARS-CoV-2 S protein: ACE2 interaction reveals novel allosteric targets. eLife. 2021; 10. doi: 10.7554/elife.63646
[23]Belouzard S, Millet JK, Licitra BN, et al. Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein. Viruses. 2012; 4(6): 1011-1033. doi: 10.3390/v4061011
[24]Bosch BJ, Smits SL, Haagmans BL. Membrane ectopeptidases targeted by human coronaviruses. Current Opinion in Virology. 2014; 6: 55-60. doi: 10.1016/j.coviro.2014.03.011
[25]Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences. 2020; 117(21): 11727-11734. doi: 10.1073/pnas.2003138117
[26]Harrison SC. Mechanism of membrane fusion by viral envelope proteins. Adv Virus Res. 2005; 64: 231-261. doi: 10.1016/S0065-3527(05)64007-9. PMID: 16139596.
[27]Koppisetti RK, Fulcher YG, Van Doren SR. Fusion Peptide of SARS-CoV-2 Spike Rearranges into a Wedge Inserted in Bilayered Micelles. Journal of the American Chemical Society. 2021; 143(33): 13205-13211. doi: 10.1021/jacs.1c05435
[28]Simmons G, Zmora P, Gierer S, et al. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. Antiviral Research. 2013; 100(3): 605-614. doi: 10.1016/j.antiviral.2013.09.028
[29]Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proceedings of the National Academy of Sciences. 2014; 111(42): 15214-15219. doi: 10.1073/pnas.1407087111
[30]Takeda M. Proteolytic activation of SARS‐CoV‐2 spike protein. Microbiology and Immunology. 2021; 66(1): 15-23. doi: 10.1111/1348-0421.12945
[31]Bertram S, Glowacka I, Müller MA, et al. Cleavage and Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by Human Airway Trypsin-Like Protease. Journal of Virology. 2011; 85(24): 13363-13372. doi: 10.1128/jvi.05300-11
[32]Chan YA, Zhan SH. The Emergence of the Spike Furin Cleavage Site in SARS-CoV-2. Kumar S, ed. Molecular Biology and Evolution. 2021; 39(1). doi: 10.1093/molbev/msab327
[33]Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Molecular Cell. 2020; 78(4): 779-784. doi: 10.1016/j.molcel.2020.04.022
[34]Jawad B, Adhikari P, Podgornik R, et al. Key Interacting Residues between RBD of SARS-CoV-2 and ACE2 Receptor: Combination of Molecular Dynamics Simulation and Density Functional Calculation. Journal of Chemical Information and Modeling. 2021; 61(9): 4425-4441. doi: 10.1021/acs.jcim.1c00560
[35]Carvalho PPD, Alves NA. Featuring ACE2 binding SARS-CoV and SARS-CoV-2 through a conserved evolutionary pattern of amino acid residues. Journal of Biomolecular Structure and Dynamics. 2021; 40(22): 11719-11728. doi: 10.1080/07391102.2021.1965028
[36]Yerukala Sathipati S, Shukla SK, Ho SY. Tracking the amino acid changes of spike proteins across diverse host species of severe acute respiratory syndrome coronavirus 2. iScience. 2022; 25(1): 103560. doi: 10.1016/j.isci.2021.103560
[37]Zhai X, Sun J, Yan Z, et al. Comparison of Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Binding to ACE2 Receptors from Human, Pets, Farm Animals, and Putative Intermediate Hosts. Gallagher T, ed. Journal of Virology. 2020; 94(15). doi: 10.1128/jvi.00831-20
[38]Nour AM, Li Y, Wolenski J, et al. Viral Membrane Fusion and Nucleocapsid Delivery into the Cytoplasm are Distinct Events in Some Flaviviruses. Pierson TC, ed. PLoS Pathogens. 2013; 9(9): e1003585. doi: 10.1371/journal.ppat.1003585
[39]V’kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology. 2020; 19(3): 155-170. doi: 10.1038/s41579-020-00468-6
[40]Ahlquist P, Noueiry AO, Lee WM, et al. Host Factors in Positive-Strand RNA Virus Genome Replication. Journal of Virology. 2003; 77(15): 8181-8186. doi: 10.1128/jvi.77.15.8181-8186.2003
[41]Upadhyay M, Gupta S. Endoplasmic reticulum secretory pathway: Potential target against SARS-CoV-2. Virus Research. 2022; 320: 198897. doi: 10.1016/j.virusres.2022.198897
[42]Scherer KM, Mascheroni L, Carnell GW, et al. SARS-CoV-2 nucleocapsid protein adheres to replication organelles before viral assembly at the Golgi/ERGIC and lysosome-mediated egress. Science Advances. 2022; 8(1). doi: 10.1126/sciadv.abl4895
[43]Siu YL, Teoh KT, Lo J, et al. The M, E, and N Structural Proteins of the Severe Acute Respiratory Syndrome Coronavirus Are Required for Efficient Assembly, Trafficking, and Release of Virus-Like Particles. Journal of Virology. 2008; 82(22): 11318-11330. doi: 10.1128/jvi.01052-08
[44]Villanueva RA, Rouillé Y, Dubuisson J. Interactions between virus proteins and host cell membranes during the viral life cycle. Int Rev Cytol. 2005; 245: 171-244. doi: 10.1016/S0074-7696(05)45006-8. PMID: 16125548
[45]Seltzer S. Linking ACE2 and angiotensin II to pulmonary immunovascular dysregulation in SARS-CoV-2 infection. International Journal of Infectious Diseases. 2020; 101: 42-45. doi: 10.1016/j.ijid.2020.09.041
[46]Burrell LM, Johnston CI, Tikellis C, et al. ACE2, a new regulator of the renin–angiotensin system. Trends in Endocrinology & Metabolism. 2004; 15(4): 166-169. doi: 10.1016/j.tem.2004.03.001
[47]Silhol F, Sarlon G, Deharo JC, et al. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: should we block the renin–angiotensin system? Hypertension Research. 2020; 43(8): 854-856. doi: 10.1038/s41440-020-0476-3
[48]Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? American Journal of Physiology-Heart and Circulatory Physiology. 2016; 310(2): H137-H152. doi: 10.1152/ajpheart.00618.2015
[49]Tamura K, Wakui H, Azushima K, et al. Angiotensin II Type 1 Receptor Binding Molecule ATRAP as a Possible Modulator of Renal Sodium Handling and Blood Pressure in Pathophysiology. Current Medicinal Chemistry. 2015; 22(28): 3210-3216. doi: 10.2174/0929867322666150821095036
[50]Blaustein MP, Leenen FHH, Chen L, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 2012; 302(5): H1031-H1049. doi: 10.1152/ajpheart.00899.2011
[51]Pratiwi A, Hakim TR, Abidin MZ, et al. Angiotensin-converting enzyme inhibitor activity of peptides derived from Kacang goat skin collagen through thermolysin hydrolysis. January-2021. 2021; 14(1): 161-167. doi: 10.14202/vetworld.2021.161-167
[52]Karnik SS, Singh KD, Tirupula K, et al. Significance of angiotensin 1–7 coupling with MAS1 receptor and other GPCRs to the renin‐angiotensin system: IUPHAR Review 22. British Journal of Pharmacology. 2017; 174(9): 737-753. doi: 10.1111/bph.13742
[53]Santos RA. Angiotensin-(1–7). Hypertension. 2014; 63(6): 1138-1147. doi: 10.1161/hypertensionaha.113.01274
[54]Bosso M, Thanaraj TA, Abu-Farha M, et al. The Two Faces of ACE2: The Role of ACE2 Receptor and Its Polymorphisms in Hypertension and COVID-19. Molecular Therapy - Methods & Clinical Development. 2020; 18: 321-327. doi: 10.1016/j.omtm.2020.06.017
[55]Valente J, António J, Mora C, et al. Developments in Image Processing Using Deep Learning and Reinforcement Learning. Journal of Imaging. 2023; 9(10): 207. doi: 10.3390/jimaging9100207
[56]Pudjihartono N, Fadason T, Kempa-Liehr AW, et al. A Review of Feature Selection Methods for Machine Learning-Based Disease Risk Prediction. Frontiers in Bioinformatics. 2022; 2. doi: 10.3389/fbinf.2022.927312
[57]De Teyou GK, Tarabalka Y, Manighetti I, et al. Deep Neural Networks for automatic extraction of features in time series satellite images. Available online: https://arxiv.org/abs/2008.08432 (accessed on 17 May 2024).
[58]Sodhani S, Faramarzi M, Mehta SV, et al. An Introduction to Lifelong Supervised Learning. Available online: https://arxiv.org/abs/2207.04354 (accessed on 17 May 2024).
[59]Yang R. Unsupervised machine learning for physical concepts. Available online: https://arxiv.org/abs/2205.05279 (accessed on 17 May 2024).
[60]Goel D, Neumann A, Neumann F, et al. Evolving Reinforcement Learning Environment to Minimize Learner’s Achievable Reward: An Application on Hardening Active Directory Systems. Available online: https://arxiv.org/abs/2304.03998 (accessed on 17 May 2024).
[61]Chitnis R, Xu Y, Hashemi B, et al. IQL-TD-MPC: Implicit Q-Learning for Hierarchical Model Predictive Control. Available online: https://arxiv.org/abs/2306.00867 (accessed on 17 May 2024).
[62]Neufeld A, Sester J. Robust Q-learning Algorithm for Markov Decision Processes under Wasserstein Uncertainty. Available online: https://arxiv.org/abs/2210.00898 (accessed on 17 May 2024)
[63]Ronecker MP, Zhu Y. Deep Q-Network Based Decision Making for Autonomous Driving. Available online: https://arxiv.org/abs/2303.11634 (accessed on 17 May 2024).
[64]Kadurin A, Nikolenko S, Khrabrov K, et al. druGAN: An Advanced Generative Adversarial Autoencoder Model for de Novo Generation of New Molecules with Desired Molecular Properties in Silico. Molecular Pharmaceutics. 2017; 14(9): 3098-3104. doi: 10.1021/acs.molpharmaceut.7b00346
[65]Dai W, Guo D. A Ligand-Based Virtual Screening Method Using Direct Quantification of Generalization Ability. Molecules. 2019; 24(13): 2414. doi: 10.3390/molecules24132414
[66]Maia EHB, Assis LC, de Oliveira TA, et al. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Frontiers in Chemistry. 2020; 8. doi: 10.3389/fchem.2020.00343
[67]Tran-Nguyen VK, Junaid M, Simeon S, et al. A practical guide to machine-learning scoring for structure-based virtual screening. Nature Protocols. 2023; 18(11): 3460-3511. doi: 10.1038/s41596-023-00885-w
[68]Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020; 10(5): 766-788. doi: 10.1016/j.apsb.2020.02.008
[69]Fassihi A, Hatami S, Sirous H, et al. Preparing a database of corrected protein structures important in cell signaling pathways. Research in Pharmaceutical Sciences. 2023; 18(1): 67. doi: 10.4103/1735-5362.363597
[70]Revillo Imbernon J, Chiesa L, Kellenberger E. Mining the Protein Data Bank to inspire fragment library design. Frontiers in Chemistry. 2023; 11. doi: 10.3389/fchem.2023.1089714
[71]Junk P, Kiel C. HOMELETTE: a unified interface to homology modelling software. Valencia A, ed. Bioinformatics. 2021; 38(6): 1749-1751. doi: 10.1093/bioinformatics/btab866
[72]Yang Z, Zeng X, Zhao Y, et al. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduction and Targeted Therapy. 2023; 8(1). doi: 10.1038/s41392-023-01381-z
[73]Konc J, Janežič D. Protein binding sites for drug design. Biophysical Reviews. 2022; 14(6): 1413-1421. doi: 10.1007/s12551-022-01028-3
[74]Alzyoud L, Bryce RA, Al Sorkhy M, et al. Structure-based assessment and druggability classification of protein–protein interaction sites. Scientific Reports. 2022; 12(1). doi: 10.1038/s41598-022-12105-8
[75]Piazza I, Beaton N, Bruderer R, et al. A machine learning-based chemoproteomic approach to identify drug targets and binding sites in complex proteomes. Nature Communications. 2020; 11(1). doi: 10.1038/s41467-020-18071-x
[76]Rufer AC. Drug discovery for enzymes. Drug Discovery Today. 2021; 26(4): 875-886. doi: 10.1016/j.drudis.2021.01.006
[77]Farooq Q ul A, Shaukat Z, Aiman S, et al. Protein-protein interactions: Methods, databases, and applications in virus-host study. World Journal of Virology. 2021; 10(6): 288-300. doi: 10.5501/wjv.v10.i6.288
[78]Matthew AN, Leidner F, Lockbaum GJ, et al. Drug Design Strategies to Avoid Resistance in Direct-Acting Antivirals and Beyond. Chemical Reviews. 2021; 121(6): 3238-3270. doi: 10.1021/acs.chemrev.0c00648
[79]Wang Y, Wei Z, Xi L. Sfcnn: a novel scoring function based on 3D convolutional neural network for accurate and stable protein–ligand affinity prediction. BMC Bioinformatics. 2022; 23(1). doi: 10.1186/s12859-022-04762-3
[80]Kosugi T, Ohue M. Quantitative Estimate Index for Early-Stage Screening of Compounds Targeting Protein-Protein Interactions. International Journal of Molecular Sciences. 2021; 22(20): 10925. doi: 10.3390/ijms222010925
[81]Eberhardt J, Santos-Martins D, Tillack AF, et al. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. Journal of Chemical Information and Modeling. 2021; 61(8): 3891-3898. doi: 10.1021/acs.jcim.1c00203
[82]Ropp PJ, Kaminsky JC, Yablonski S, et al. Dimorphite-DL: an open-source program for enumerating the ionization states of drug-like small molecules. Journal of Cheminformatics. 2019; 11(1). doi: 10.1186/s13321-019-0336-9
[83]Nash S, Vachet RW. Gas-Phase Unfolding of Protein Complexes Distinguishes Conformational Isomers. Journal of the American Chemical Society. 2022; 144(48): 22128-22139. doi: 10.1021/jacs.2c09573
[84]Cleves AE, Jain AN. Structure- and Ligand-Based Virtual Screening on DUD-E+: Performance Dependence on Approximations to the Binding Pocket. Journal of Chemical Information and Modeling. 2020; 60(9): 4296-4310. doi: 10.1021/acs.jcim.0c00115
[85]Tang S, Chen R, Lin M, et al. Accelerating AutoDock Vina with GPUs. Molecules. 2022; 27(9): 3041. doi: 10.3390/molecules27093041
[86]Jumper J, Hassabis D. Protein structure predictions to atomic accuracy with AlphaFold. Nature Methods. 2022; 19(1): 11-12. doi: 10.1038/s41592-021-01362-6
[87]Chen T, Shu X, Zhou H, et al. Algorithm selection for protein–ligand docking: strategies and analysis on ACE. Scientific Reports. 2023; 13(1). doi: 10.1038/s41598-023-35132-5
[88]Mohammadi S, Narimani Z, Ashouri M, et al. Ensemble learning from ensemble docking: revisiting the optimum ensemble size problem. Scientific Reports. 2022; 12(1). doi: 10.1038/s41598-021-04448-5
[89]Verburgt J, Kihara D. Benchmarking of structure refinement methods for protein complex models. Proteins: Structure, Function, and Bioinformatics. 2021; 90(1): 83-95. doi: 10.1002/prot.26188
[90]Peivaste I, Ramezani S, Alahyarizadeh G, et al. Rapid and accurate predictions of perfect and defective material properties in atomistic simulation using the power of 3D CNN-based trained artificial neural networks. Scientific Reports. 2024; 14(1). doi: 10.1038/s41598-023-50893-9
[91]Aziz S, Waqas M, Mohanta TK, et al. Identifying non-nucleoside inhibitors of RNA-dependent RNA-polymerase of SARS-CoV-2 through per-residue energy decomposition-based pharmacophore modeling, molecular docking, and molecular dynamics simulation. Journal of Infection and Public Health. 2023; 16(4): 501-519. doi: 10.1016/j.jiph.2023.02.009
[92]Gazi R, Maity S, Jana M. Conformational Features and Hydration Dynamics of Proteins in Cosolvents: A Perspective from Computational Approaches. ACS Omega. 2023; 8(3): 2832-2843. doi: 10.1021/acsomega.2c08009
[93]Wang X, Chong B, Sun Z, et al. More is simpler: Decomposition of ligand‐binding affinity for proteins being disordered. Protein Science. 2022; 31(7). doi: 10.1002/pro.4375
[94]Kurniawan J, Ishida T. Protein Model Quality Estimation Using Molecular Dynamics Simulation. ACS Omega. 2022; 7(28): 24274-24281. doi: 10.1021/acsomega.2c01475
[95]Li Y, Hou S, Zhang Y, et al. Effect of Travel Restrictions of Wuhan City Against COVID-19: A Modified SEIR Model Analysis. Disaster Medicine and Public Health Preparedness. 2021; 16(4): 1431-1437. doi: 10.1017/dmp.2021.5
[96]Chakraborty M, Shakir Mahmud M, Gates TJ, et al. Analysis and Prediction of Human Mobility in the United States during the Early Stages of the COVID-19 Pandemic using Regularized Linear Models. Transportation Research Record: Journal of the Transportation Research Board. 2022; 2677(4): 380-395. doi: 10.1177/03611981211067794
[97]Samad A, Ajmal A, Mahmood A, et al. Identification of novel inhibitors for SARS-CoV-2 as therapeutic options using machine learning-based virtual screening, molecular docking and MD simulation. Frontiers in Molecular Biosciences. 2023; 10. doi: 10.3389/fmolb.2023.1060076
[98]Zhang L, Zhao H, Liu J, et al. Design of SARS-CoV-2 Mpro, PLpro Dual-Target Inhibitors Based on Deep Reinforcement Learning and Virtual Screening. Future Medicinal Chemistry. 2022; 14(6): 393-405. doi: 10.4155/fmc-2021-0269
[99]Higgins MK. Can We AlphaFold Our Way Out of the Next Pandemic? Journal of Molecular Biology. 2021; 433(20): 167093. doi: 10.1016/j.jmb.2021.167093
[100]Ismi DP, Pulungan R, Afiahayati. Deep learning for protein secondary structure prediction: Pre and post-AlphaFold. Computational and Structural Biotechnology Journal. 2022; 20: 6271-6286. doi: 10.1016/j.csbj.2022.11.012
[101]Marcu ŞB, Tăbîrcă S, Tangney M. An Overview of Alphafold’s Breakthrough. Frontiers in Artificial Intelligence. 2022; 5. doi: 10.3389/frai.2022.875587
[102]Bertoline LMF, Lima AN, Krieger JE, et al. Before and after AlphaFold2: An overview of protein structure prediction. Frontiers in Bioinformatics. 2023; 3. doi: 10.3389/fbinf.2023.1120370
[103]DeBenedictis EA, Chory EJ, Gretton DW, et al. Systematic molecular evolution enables robust biomolecule discovery. Nature Methods. 2021; 19(1): 55-64. doi: 10.1038/s41592-021-01348-4
[104]Xu YC, ShangGuan TJ, Ding XM, et al. Accurate prediction of protein torsion angles using evolutionary signatures and recurrent neural network. Scientific Reports. 2021; 11(1). doi: 10.1038/s41598-021-00477-2
[105]Kilim O, Mentes A, Pál B, et al. SARS-CoV-2 receptor-binding domain deep mutational AlphaFold2 structures. Scientific Data. 2023; 10(1). doi: 10.1038/s41597-023-02035-z
[106]Gutnik D, Evseev P, Miroshnikov K, Shneider M. Using AlphaFold Predictions in Viral Research. Curr Issues Mol Biol. 2023; 45: 3705-3732. doi: 10.3390/cimb45040240
[107]Ali MA, Caetano-Anollés G. AlphaFold2 Reveals Structural Patterns of Seasonal Haplotype Diversification in SARS-CoV-2 Spike Protein Variants. Biology. 2024; 13(3): 134. doi: 10.3390/biology13030134
[108]Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021; 596(7873): 583-589. doi: 10.1038/s41586-021-03819-2
[109]Lv H, Shi L, Berkenpas JW, et al. Application of artificial intelligence and machine learning for COVID-19 drug discovery and vaccine design. Brief Bioinform. 2021; 22: 320. doi: 10.1093/bib/bbab320
[110]Kalita P, Tripathi T, Padhi AK. Computational Protein Design for COVID-19 Research and Emerging Therapeutics. ACS Central Science. 2023; 9(4): 602-613. doi: 10.1021/acscentsci.2c01513
[111]Li J, McKay KT, Remington JM, et al. A computational study of cooperative binding to multiple SARS-CoV-2 proteins. Scientific Reports. 2021; 11(1). doi: 10.1038/s41598-021-95826-6
[112]Ashique S, Mishra N, Mohanto S, et al. Application of artificial intelligence (AI) to control COVID-19 pandemic: Current status and future prospects. Heliyon. 2024; 10(4): e25754. doi: 10.1016/j.heliyon.2024.e25754
[113]Prasad K, Kumar V. Artificial intelligence-driven drug repurposing and structural biology for SARS-CoV-2. Current Research in Pharmacology and Drug Discovery. 2021; 2: 100042. doi: 10.1016/j.crphar.2021.100042
[114]Keshavarzi Arshadi A, Webb J, Salem M, et al. Artificial Intelligence for COVID-19 Drug Discovery and Vaccine Development. Frontiers in Artificial Intelligence. 2020; 3. doi: 10.3389/frai.2020.00065
[115]Ghosh A, Larrondo-Petrie MM, Pavlovic M. Revolutionizing Vaccine Development for COVID-19: A Review of AI-Based Approaches. Information. 2023; 14(12): 665. doi: 10.3390/info14120665
[116]Wang L, Zhang Y, Wang D, et al. Artificial Intelligence for COVID-19: A Systematic Review. Frontiers in Medicine. 2021; 8. doi: 10.3389/fmed.2021.704256
Copyright (c) 2024 Yasunari Matsuzaka, Ryu Yashiro

This work is licensed under a Creative Commons Attribution 4.0 International License.