Mathematical modelling of transmission dynamics of Dengue Fever in the presence of infective immigrants
Abstract
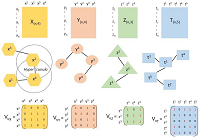
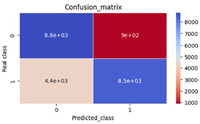
Dengue fever is one of the neglected tropical diseases around the globe and its ravaging effect over the period has been enormous in the affected areas. Globalisation, immigration and urbanization and poor urban planning have become the contributory factors in the spread of infectious diseases. In this paper, a model describing the dynamics of dengue fever incorporated with infection immigrants is formulated and analysed using ordinary differential equations with a constant immigration recruitment rate. The model was qualitatively and quantitatively analysed for its local stability, basic reproductive number and sensitivity of the model parameters values to the basic reproductive number to understand the impact of the parameters on the disease spread. In the analysis, it was found that in the presence of infectious immigrants, there cannot be a disease free state demonstrated by ∅ ≥ 0 where the model demonstrates a unique endemic equilibrium state if the fraction of infectious immigrants ∅ is positive. The unique endemic equilibrium for which there is a fraction of infectious immigrants is globally asymptotically stable. Numerical simulation was performed and the results displayed graphically and discussed. It was revealed that immigration of infected immigrants contributes significantly in the spread of dengue fever and that it can be controlled by preventing the influx of infected immigrants and reducing the mosquitoes and human contact rate.
References
[1]Nuraini N, Tasman H, Soewono E, et al. A with-in host Dengue infection model with immune response. Mathematical and Computer Modelling. 2009; 49(5-6): 1148-1155. doi: 10.1016/j.mcm.2008.06.016
[2]Sirisena PDNN, Noordeen F. Evolution of dengue in Sri Lanka—changes in the virus, vector, and climate. International Journal of Infectious Diseases. 2014; 19: 6-12. doi: 10.1016/j.ijid.2013.10.012
[3]Amarasinghe A. Dengue Virus Infection in Africa. Emerging Infectious Diseases. Published online August 2011. doi: 10.3201/eid1708.101515
[4]World Health Organization. Dengue and severe dengue. World Health Organization; 2016.
[5]World Health Organization, Special Programme for Research, Training in Tropical Diseases, World Health Organization. Department of Control of Neglected Tropical Diseases, World Health Organization. Epidemic, and Pandemic Alert. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization; 2009.
[6]Garba SM, Gumel AB, Abu Bakar MR. Backward bifurcations in dengue transmission dynamics. Mathematical Biosciences. 2008; 215(1): 11-25. doi: 10.1016/j.mbs.2008.05.002
[7]Brady OJ, Gething PW, Bhatt S, et al. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Neglected Tropical Diseases. 2012; 6(8): e1760. doi: 10.1371/journal.pntd.0001760
[8]Osman S, Omari-Sasu AY, Boadi RK. Logit model for the determinants of drug driving in Ghana. International Journal of Statistics and Application. 2016; 6(6): 339-346.
[9]Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013; 496(7446): 504-507. doi: 10.1038/nature12060
[10]Phaijoo GR, Gurung DB. Modeling Impact of Temperature and Human Movement on the Persistence of Dengue Disease. Computational and Mathematical Methods in Medicine. 2017; 2017: 1-9. doi: 10.1155/2017/1747134
[11]Osman S, Otoo D, Sebil C, Makinde OD. Bifurcation, sensitivity and optimal control analysis of modelling anthrax-listeriosis co-dynamics. Commun. Math. Biol. Neurosci. 2020.
[12]Tilahun GT, Makinde OD, Malonza D. Modelling and optimal control of pneumonia disease with cost-effective strategies. Journal of Biological Dynamics. 2017; 11(sup2): 400-426. doi: 10.1080/17513758.2017.1337245
[13]Otoo D, Edusei H, Gyan A, et al. Optimal prevention of HIV-AIDS with emphasis on unprotected and unnatural canal activities: a deterministic modelling perspective. Commun. Math. Biol. Neurosci. 2023.
[14]Okosun KO, Makinde OD. A co-infection model of malaria and cholera diseases with optimal control. Mathematical Biosciences. 2014; 258: 19-32. doi: 10.1016/j.mbs.2014.09.008
[15]Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environment International. 2019; 130: 104909. doi: 10.1016/j.envint.2019.104909
[16]Ndii, M. Z., Anggriani, N., Messakh, J. J., & Djahi, B. S. (2021). Estimating the reproduction number and designing the integrated strategies against dengue. Results in Physics, 27, 104473.
[17]O’Reilly, K. M., Hendrickx, E., Kharisma, D. D., Wilastonegoro, N. N., Carrington, L. B., Elyazar, I. R., ... & Brady, O. J. (2019). Estimating the burden of dengue and the impact of release of wMel Wolbachia-infected mosquitoes in Indonesia: a modelling study. BMC medicine, 17, 1-14.
[18]Tilahun GT, Makinde OD, Malonza D. Modelling and Optimal Control of Typhoid Fever Disease with Cost-Effective Strategies. Computational and Mathematical Methods in Medicine. 2017; 2017: 1-16. doi: 10.1155/2017/2324518
[19]Tilahun GT. Modeling co-dynamics of pneumonia and meningitis diseases. Advances in Difference Equations. 2019; 2019(1). doi: 10.1186/s13662-019-2087-3
[20]Karanja TW, Osman S, Wainaina M. Analysis and Modelling of Ringworm Infections in an Environment. Global Journal of Pure and Applied Mathematics. 2019; 15(5): 649-665.
[21]Onsongo WM, Mwini ED, Nyanaro BN, Osman S. The dynamics of psittacosis in human and poultry populations: a mathematical modelling perspective. J. Math. Comput. Sci. 2021; 11(6): 8472-8505.
Copyright (c) 2024 Elvis Kobina Donkoh, Dominic Otoo, Shaibu Osman, Maxwell Baafi, Martin Anokye, Ernest Yeboah Boateng

This work is licensed under a Creative Commons Attribution 4.0 International License.