Synthesis of MgO nanoparticles for different annealing temperatures and its biomedical applications
Abstract
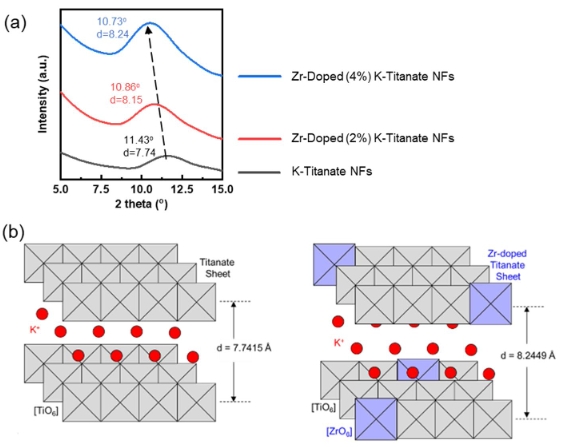
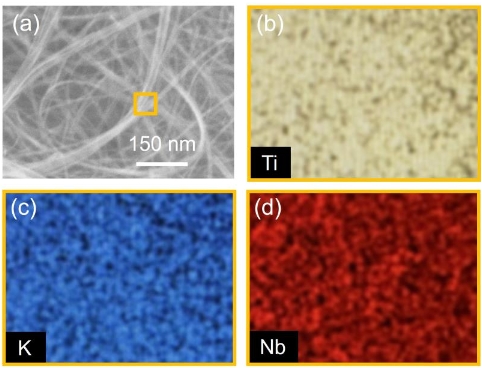
In this paper, we report the synthesis of MgO nanoparticles (NPs) by the co-precipitation method. The structural properties of the samples were characterised by X-ray diffraction, which revealed that the MgO Nps have a cubic structure. The functional groups of the as-synthesised samples were analysed by Fourier transform infrared spectroscopy. The optical properties of the as-synthesised samples were studied by UV-vis spectroscopy in the range of 200–800 nm, and the energy bandgap was calculated by the taus relation. The magnesium oxide (MgO) nanoparticles (NPs) showed significant dose-dependent bactericidal activity in both gram-negative and gram-positive bacteria. From the analysis of the antibacterial and antifungal activities of MgO NPs, it is revealed that the dose is sufficient for killing. These may be used in medical applications.
References
[1]Sekar P, Narendranath S, Desai V. Recent progress in in vivo studies and clinical applications of magnesium based biodegradable implants—A review. Journal of Magnesium and Alloys 2021; 9(4): 1147–1163. doi: 10.1016/j.jma.2020.11.001.
[2]Nie Y, Dai J, Li X, Zhang X. Recent developments on corrosion behaviors of Mg alloys with stacking fault or long period stacking ordered structures. Journal of Magnesium and Alloys 2021; 9(4): 1123–1146. doi: 10.1016/j.jma.2020.09.021.
[3]Molaei M, Babaei K, Fattah-alhosseini A. Improving the wear resistance of plasma electrolytic oxidation (PEO) coatings applied on Mg and its alloys under the addition of nano- and micro-sized additives into the electrolytes: A review. Journal of Magnesium and Alloys 2021; 9(4): 1164–1186. doi: 10.1016/j.jma.2020.11.016.
[4]Wang C, Kang J, Deng K, et al. Microstructure and mechanical properties of Mg-4Zn-xGd (x=0, 0.5, 1, 2) alloys. Journal of Magnesium and Alloys 2020; 8(2): 441–451. doi: 10.1016/j.jma.2019.06.005.
[5]Ariga K, Yamauchi Y, Aono M. Commentary: Nanoarchitectonics—Think about NANO again. APL Materials 2015; 3(6): 061001. doi: 10.1063/1.4922549.
[6]Bdewi SF, Abdullah OG, Aziz BK, Muta AAR. Synthesis, structural and optical characterization of MgO nanocrystalline embedded in PVA matrix. Journal of Inorganic and Organometallic Polymers and Materials 2016; 26: 326–334. doi: 10.1007/s10904-015-0321-3.
[7]Tamilselvi P, Yelilarasi A, Hema M, Anbarasan R. Synthesis of hierarchical structured MgO by sol-gel method. Journal of Nanotechnology Bulletin 2013; 2(1): 130106–1301065. doi: 10.1234/NANO130106.
[8]Guan H, Wang P, Zhao B, et al. Synthesis of high surface area nanometer magnesia by solid-state chemical reaction. Frontiers of Chemistry in China 2007; 2: 204–208. doi: 10.1007/s11458-007-0041-5.
[9]Niu H, Yang Q, Tang K, Xie Y. Large-scale synthesis of single-crystalline MgO with bone-like nanostructures. Journal of Nanoparticle Reasearch 2006; 8: 881–888. doi: 10.1007/s11051-006-9138-x.
[10]Yuan G, Zheng J, Lin C, et al. Electrosynthesis and catalytic properties of magnesium oxide nanocrystals with porous structures. Materials Chemistry and Physics 2011; 130(1–2): 387–391. doi: 10.1016/j.matchemphys.2011.06.058.
[11]Wagner GW, Bartram PW, Koper O, Klabunde KJ. Reactions of VX, GD, and HD with nanosize MgO. The Journal of Physical Chemistry B 1999; 103(16): 3225–3228. doi: 10.1021/jp984689u.
[12]Moorthy SK, Ashok CH, Venkateswara Rao V, Viswanathan C. Synthesis and characterization of Mgo nanoparticles by neem leaves through green method. Materials Today: Proceedings 2015; 2(9): 4360–4368. doi: 10.1016/j.matpr.2015.10.027.
[13]Park JS, Han YH. Effects of MgO coating on microstructure and dielectric properties of BaTiO3. Journal of the European Ceramic Society 2007; 27(2–3):1077–1082. doi: 10.1016/j.jeurceramsoc.2006.05.073.
[14]Salem JK, El-Nahhal IM, Hammad TM, et al. Optical and fluorescence properties of MgO nanoparticles in micellar solution of hydroxyethyl laurdimonium chloride. Chemical Physics Letters 2015; 636: 26–30. doi: 10.1016/j.cplett.2015.07.014.
[15]Najafi A. A novel synthesis method of hierarchical mesoporous MgO nanoflakes employing carbon nanoparticles as the hard templates for photocatalytic degradation. Ceramics International 2017; 43(7): 5813–5818. doi: 10.1016/j.ceramint.2017.01.135.
[16]Sutradhar N, Sinhamahapatra A, Pahari SK, et al. Controlled synthesis of different morphologies of MgO and their use as solid base catalysts. The Journal of Physical Chemistry C 2011; 115(25): 12308–12316. doi: 10.1021/jp2022314.
[17]Huang CH, Jan YL, Lee WC. Investigation of Mg(O,OH) films prepared by chemical bath deposition as buffer layers for Cu(In,Ga)Se2 solar cells. Journal of the Electrochemical Society 2011; 158(9): H879. doi: 10.1149/1.3609047.
[18]Das PS, Dey A, Mandal AK, et al. Synthesis of Mg(OH)2 micro/nano flowers at room temperature. Journal of Advanced Ceramics 2013; 2(2): 173–179. doi: 10.1007/s40145-013-0058-9.
[19]Tanabe K. Solid acids and bases: Their catalytic properties. New York: Academic Press; 1970. doi: 10.1016/B978-0-12-683250-1.X5001-9.
[20]Borhede AV, Kanade KG, Tope DR, Patil MD. A comparative study on synthesis, characterization and photocatalytic activities of MgO and Fe/MgO nanoparticles. Research on Chemical Intermediates 2012; 38(8): 1931–1946. doi: 10.1007/s11164-012-0515-z.
[21]Anpo M, Moon SC, Chiba K, et al. Intrinsic surface structures and their roles in the catalysis and photo-catalysis of microcrystalline MgO catalysts. Research on Chemical Intermediates 1993; 19(6): 495–519. doi: 10.1163/156856793X00451.
[22]Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002;18(17): 6679–6686. doi: 10.1021/la0202374.
[23]Makhluf S, Dror R, Nitzan Y, et al. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Advanced Functinal Materials 2005; 15(10): 1708–1715. doi: 10.1002/adfm.200500029.
[24]Das B, Moumita S, Ghosh S, et al. Biosynthesis of magnesium oxide (MgO) nanoflakes by using leaf extract of Bauhinia purpurea and evaluation of its antibacterial property against Staphylococcus aureus. Materials Science and Engineering: C 2018; 91: 436–444. doi: 10.1016/j.msec.2018.05.059.
[25]Sawai J, Kojima H, Igarashi H, et al. Antibacterial characteristics of magnesium oxide powder. World Journal of Microbiology and Biotechnology 2000; 16(2): 187–194. doi: 10.1023/A:1008916209784.
[26]Coutinho ML, Miller AZ, Phillip A, et al. Biodeterioration of majolica glazed tiles by the fungus Devriesia imbrexigena. Construction and Building Materials 2019; 212: 49–56. doi: 10.1016/j.conbuildmat.2019.03.268.
[27]Amaral LF, Oliveira IR, Salomao R, et al. Temperature and common-ion effect on magnesium oxide (MgO) hydration. Ceramics International 2010; 36(3): 1047–1054. doi: 10.1016/j.ceramint.2009.12.009.
[28]Hall RJ, Spencer DRF. A review of the production and properties of sea-water magnesia. Interceram 1973; 22: 212–218.
[29]Mastuli MS, Kamarulzaman N, Nawawi MA, et al. Growth mechanisms of MgO nanocrystals via a sol-gel synthesis using different complexing agents. Nanoscale Research Letters 2014; 9: 134. doi: 10.1186/1556-276X-9-134.
[30]Mehta M, Mukhopadhyay M, Christian R, Mistry N. Synthesis and characterization of MgO nanocrystals using strong and weak bases. Powder Technology 2012; 226: 213–221. doi: 10.1016/j.powtec.2012.04.044.
[31]Bokhimi, Morales A, Lopez T, Gomez R. Crystalline structure of MgO prepared by the sol-gel technique with different hydrolysis catalysts. Journal of Solid State Chemistry 1995; 115(2): 411–415. doi: 10.1006/jssc.1995.1152.
[32]Ma J, Chen CZ, Wang DG, Hu JH. Synthesis, characterization and in vitro bioactivity of magnesium-doped sol-gel glass and glass-ceramics. Ceramics International 2011; 37(5): 1637–1644. doi: 10.1016/j.ceramint.2011.01.043.
[33]Portillo R, Lopez T, Gomez R, et al. Magnesia synthesis via sol-gel: Structure and reactivity. Lengmuir 1996; 12(1): 40–44. doi: 10.1021/la940694n.
[34]Demirci S, Ozturk B, Yildirim S, et al. Synthesis and comparison of the photocatalytic activities of flame spray pyrolysis and sol–gel derived magnesium oxide nano-scale particles. Materials Science in Semiconductor Processing 2015; 34: 154–161. doi: 10.1016/j.mssp.2015.02.029.
[35]Mirzaei H, Davoodnia A. Microwave assisted sol-gel synthesis of MgO nanoparticles and their catalytic activity in the synthesis of hantzsch 1,4-dihydropyridines. Chinese Journal of Catalysis 2012; 33(9–10): 1502–1507. doi: 10.1016/S1872-2067(11)60431-2.
[36]Alavi MA, Morsali A. Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method. Ultrasonics Sonochemistry 2010; 17(2): 441–446. doi: 10.1016/j.ultsonch.2009.08.013.
[37]Wang JA, Novaro O, Bokhimi X, et al. Characterizations of the thermal decomposition of brucite prepared by sol-gel technique for synthesis of nanocrystalline MgO. Materials Letters 1998; 35(5–6): 317–323. doi: 10.1016/S0167-577X(97)00273-5.
[38]Li H, Li M, Wang X, et al. Synthesis and optical properties of single-crystal MgO nanobelts. Materials Letters 2013; 102–103: 80–82. doi: 10.1016/j.matlet.2013.03.118.
[39]Nagashima K, Yanagida T, Tanaka H, Kawai T. Epitaxial growth of MgO nanowires by pulsed laser deposition. Journal of Applied Physics 2007; 101(12): 124304. doi: 10.1063/1.2748625.
[40]Perry SS, Merrill PB. Preparation and characterization of MgO(100) surfaces. Surface Science 1997; 383 (2–3): 268–276. doi: 10.1016/S0039-6028(97)00185-4.
[41]Henrist C, Mathieu JP, Vogels C, et al. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. Jouranl of Crystal Growth 2003; 249(1–2): 321–330. doi: 10.1016/S0022-0248(02)02068-7.
[42]Fedorov PP, Tkachenko EA, Kuznetsov SV, et al. Inorganic Materials 2007; 43: 502–504. doi: 10.1134/S0020168507050111.
[43]Cvetkovic VS, Vukicevic NM, Nikolic ND, et al. Formation of needle-like and honeycomb-like magnesium oxide/hydroxide structures by electrodeposition from magnesium nitrate melts. Electrochimica Acta 2018; 268: 494–502. doi: 10.1016/j.electacta.2018.02.121.
[44]Yang W, Zhu Z, Shi J, et al. Characterizations of the thermal decomposition of nano-magnesium hydroxide by positron annihilation lifetime spectroscopy. Powder Technology 2017; 311: 206–212. doi: 10.1016/j.powtec.2017.01.059.
[45]Tamilselvi P, Yelilarasi A, Hema M, Anbarasan R. Synthesis of hierarchical structured MgO by sol-gel method. Nano Bulletin 2013; 2: 130106. doi: 10.1234/NANO130106.
[46]Chung KT, Wong TY, Wei CI, et al. Tannins and human health: A review. Critical Reviews in Food Science and Nutrition 1998; 38(6): 421–464. doi: 10.1080/10408699891274273.
[47]Nigam A, Saini S, Rai AK, Pawar SJ. Structural, optical, cytotoxicity, and antimicrobial properties of MgO, ZnO and MgO/ZnO nanocomposite for biomedical applications. Ceramics International 2021; 47(14): 19515–19525. doi: 10.1016/j.ceramint.2021.03.289.
[48]Pandey R, Jaffe JE, Kunz AB. Ab initio band-structure calculations for alkaline-earth oxides and sulfides. Physical Review B 1991; 43(11): 9228. doi: 10.1103/PhysRevB.43.9228.
[49]Bindhu MR, Umadevi M, KavinMicheal M, et al. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Materials Letters 2016; 166: 19–22. doi: 10.1016/j.matlet.2015.12.020.
[50]Islam I, Siddiqui AM, Hafiz AK, et al. Influence of pH and Fe doping on structural and physical properties of Mg0.95Mn0.05-xFexO (x = 0,0.04) nanoparticles. Journal of Physics and Chemistry of Solids 2019; 133: 197–202. doi: 10.1016/j.jpcs.2019.05.030.
[51]Bindhu MR, Umadevi M. Antibacterial activities of green synthesized gold nanoparticles. Materials Letters 2014; 120: 122–125. doi: 10.1016/j.matlet.2014.01.108.
[52]Jin T, He Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. Journal of Nanoparticle Research 2011; 13: 6877–6885. doi: 10.1007/s11051-011-0595-5.
[53]Singariya P, Kumar P, Mouriya KK. Antimicrobial activity of fruit coat (calyx) of withania somnifera against some multi drug resistant microbes. International Journal of Biological & Pharmaceutical Research 2012; 3(2): 252–258.
[54]Koka JA, Wani AH, Yaqub Bhat M. Evaluation of antifungal activity of magnesium oxide (MgO) and iron oxide (FeO) nanoparticles on rot causing fungi. Journal of Drug Delivery & Therapeutics 2019; 9(2-s): 173–178. doi: 10.22270/jddt.v9i2-s.2479.
[55]Essien ER, Astasie VN, Okeafor AO, Nwude DO. Biogenic synthesis of magnesium oxide nanoparticles using Manihot esculenta (Crantz) leaf extract. International Nano Letters 2020; 10: 43–48. doi: 10.1007/s40089-019-00290-w.
[56]Navalon S, Garcia H. Nanoparticles for catalysis. Nanomaterials 2016; 6(7): 123. doi: 10.3390/nano6070123.
[57]Narendhran S, Manikandan M, Baby Shakila P. Antibacterial, antioxidant properties of Solanum trilobatum and sodium hydroxide-mediated magnesium oxide nanoparticles: A green chemistry approach. Bulletin of Materials Science 2019; 42: 133. doi: 10.1007/s12034-019-1811-7.
[58]Patil UH, Gaikwad DK. Phytochemical screening and microbicidal activity of stem bark of Pterocarpus marsupium. International Journal of Pharma Sciences and Research 2011; 2(1): 36–40.
[59]Lin ST, Klabunde JK. Thermally activated magnesium oxide surface chemistry. Adsorption and decomposition of phosphorus compounds. Langmuir 1985; 1(5): 600–605. doi: 10.1021/la00065a015.
[60]Ekerdt JG, Klabunde KJ, Shapley JR, et al. Surface chemistry of organophosphorus compounds. The Journal of Physcical Chemistry 1988; 92(22): 6182–6188. doi: 10.1021/j100333a005.
[61]Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002; 18(17): 6679–6686. doi: 10.1021/la0202374.
Copyright (c) 2021 Annamalai Varathan Jaya Srinivasan, Iruson Baskaran, Balaraman Sathyaseelan, Krishnamoorthy Senthilnathan, Elayaperumal Manikandan

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it for any purpose, even commercially, under the condition that the authors are given credit. With this license, authors hold the copyright.