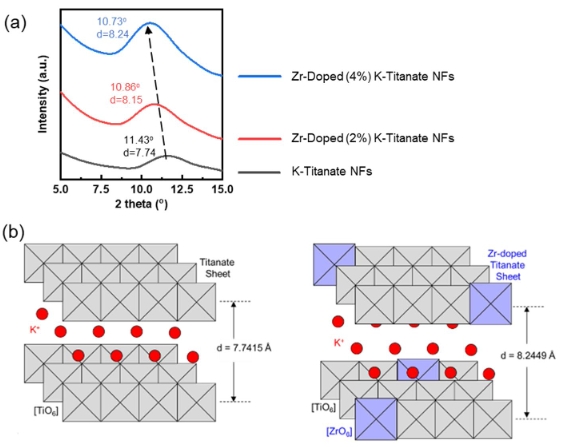
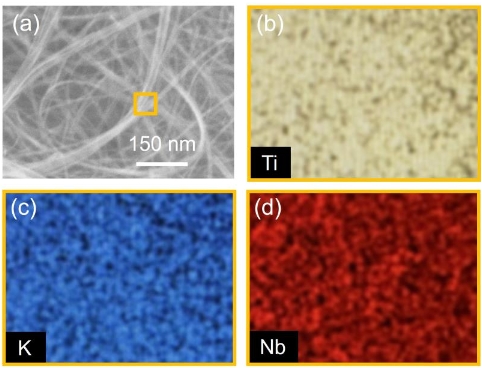
Hydrothermal synthesis of valve metal Zr-doped titanate nanofibers for bone tissue engineering
Abstract
Investigations are underway to identify novel biomaterials to improve strategies for bone tissue engineering. Hybrid nanomaterials have emerged as a viable class of biomaterials. Here, we report a facile, economical, optimized, and well-controlled hydrothermal method for synthesizing Zr-doped potassium titanate nanofibers with high purity. Upon morphological characterization, Zr-doping did not disrupt the parent crystal structure of potassium titanate, which showed huge potential for bone tissue engineering.References
[1]No YJ, Roohani-Esfahani SI, Zreiqat H. Nanomaterials: The next step in injectable bone cements. Nanomedicine 2014; 9(11): 1745–1964. doi: 10.2217/nnm.14.109
[2]Zhang B, Li J, He L, et al. Bio-surface coated titanium scaffolds with cancellous bone-like biomimetic structure for enhanced bone tissue regeneration. Acta Biomaterialia 2020; 114: 431–448. doi: 10.1016/j.actbio.2020.07.024
[3]Min Q, Liu J, Zhang Y, et al. Dual network hydrogels incorporated with bone morphogenic protein-7-loaded hyaluronic acid complex nanoparticles for inducing chondrogenic differentiation of synovium-derived mesenchymal stem cells. Pharmaceutics 2020; 12(7): 613. doi: 10.3390/pharmaceutics12070613
[4]Wu T, Li B, Wang W, et al. Strontium-substituted hydroxyapatite grown on graphene oxide nanosheet-reinforced chitosan scaffold to promote bone regeneration. Biomaterial Science 2020; 8(16): 4603–4615. doi: 10.1039/D0BM00523A
[5]Benedini L, Laiuppa J, Santillán G, et al. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Materials Science and Engineering: C 2020; 115: 111101. doi: 10.1016/j.msec.2020.111101
[6]Oudadesse H, Najem S, Mosbahi S, et al. Development of hybrid scaffold: Bioactive glass nanoparticles/chitosan for tissue engineering applications. Journal of Biomedical Materials Research Part A 2020; 109(5): 590–599. doi: 10.1002/jbm.a.37043
[7]Mallakpour S, Ramezanzade V. Green fabrication of chitosan/tragacanth gum bionanocomposite films having TiO2@Ag hybrid for bioactivity and antibacterial applications. International Journal of Biological Macromolecules 2020; 162: 512–522. doi: 10.1016/j.ijbiomac.2020.06.163
[8]Pan H, Gao H, Li Q, et al. Engineered microporous hydrogel scaffolds via pickering emulsions stabilized by MgO nanoparticles promote bone regeneration. Journal of Materials Chemistry B 2020; 8(28): 6100–6114. doi: 10.1039/D0TB00901F
[9]Nie L, Deng Y, Li P, et al. Hydroxyethyl chitosan-reinforced polyvinyl alcohol/biphasic calcium phosphate hydrogels for bone regeneration. ACS Omega 2020; 5(19): 10948–10957. doi: 10.1021/acsomega.0c00727
[10]Maji K, Dasgupta S, Bhaskar R, Gupta MK. Photo-crosslinked alginate nano-hydroxyapatite paste for bone tissue engineering. Biomedical Materials 2020; 15(5): 055019. doi: 10.1088/1748-605X/ab9551
[11]Gao C, Wei D, Yang H, et al. Nanotechnology for treating osteoporotic vertebral fractures. International Journal of Nanomedicine 2015; 10: 5139–5157. doi: 10.2147/IJN.S85037
[12]Kumar JP, Lakshmi L, Jyothsna V, et al. Synthesis and characterization of diopside particles and their suitability along with chitosan matrix for bone tissue engineering in vitro and in vivo. Journal of Biomedical Nanotechnology 2014; 10(6): 970–981. doi: 10.1166/jbn.2014.1808
[13]Saravanan S, Vimalraj S, Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomedicine & Pharmacotherapy 2018; 107: 908–917. doi: 10.1016/j.biopha.2018.08.072
[14]Mohammadi M, Mousavi Shaegh SA, Alibolandi M, et al. Micro and nanotechnologies for bone regeneration: Recent advances and emerging designs. Journal of Controlled Release 2018; 274: 35–55. doi: 10.1016/j.jconrel.2018.01.032
[15]Aldaadaa A, Al Qaysi M, Georgiou G, et al. Physical properties and biocompatibility effects of doping SiO2 and TiO2 into phosphate-based glass for bone tissue engineering. Journal of Biomaterials Applications 2018; 33(2): 271–280. doi: 10.1177/0885328218788832
[16]Hashemi A, Ezati M, Mohammadnejad J, et al. Chitosan coating of TiO2 nanotube arrays from improved metformin release and osteoblast differentiation. International Journal of Nanomedicine 2020; 15: 4471–4481. doi: 10.2147/iJN.S248927
[17]Khan S, Garg M, Chockalingam S, et al. TiO2 doped chitosan/poly (vinyl alcohol) nanocomposite film with enhanced mechanical properties for application in bone tissue regeneration. International Journal of Biological Macromolecules 2020; 143: 285–296. doi: 10.1016/j.ijbiomac.2019.11.246
[18]Liang F, Zhou L, Wang K. Apatite formation on porous titanium by alkali and heat-treatment. Surface and Coatings Technology 2003; 165: 133–139. doi: 10.1016/S0257-8972(02)00735-1
[19]Marins NH, Lee BEJ, Silva RM, et al. Niobium pentoxide and hydroxyapatite particle loaded electrospun polycaprolactone/gelatin membranes for bone tissue engineering. Colloids and Surfaces B: Biointerfaces 2019; 182: 110386. doi: 10.1016/j.colsurfb.2019.110386
[20]Antonini LM, Menezes TL, Santos AGdJ, et al. Osteogenic differentiation of bone marrow-derived mesenchymal stem cells on anodized niobium surface. Journal of Materials Science: Materials in Medicine 2019; 30(9): 104. doi: 10.1007/s10856-019-6305-z
[21]Hwang C, Park S, Kang IG, et al. Tantalum-coated polylactic acid fibrous membranes for guided bone regeneration. Materials Science and Engineering: C 2020; 115: 111112. doi: 10.1016/j.msec.2020.111112
[22]Zhang J, Huang D, Liu S, et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Materials Science and Engineering: C 2019; 105: 110054. doi: 10.1016/jmsec.2019.110054
[23]Poon KK, Wurm MC, Evans DM, et al. Biocompatibility of (Ba,Ca) (Zr,Ti)O3 piezoelectric ceramics for bone replacement materials. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2020; 108(4): 1295–1303. doi: 10.1002/jbm.b.34477
[24]Inui T, Haneda S, Sasaki M, et al. Enhanced chondrogenic differentiation of equine bone marrow-derived mesenchymal stem cells in zirconia microwell substrata. Research in Veterinary Science 2019; 125: 345–350. doi: 10.1016/j.rvsc.2019.07.005
[25]Soon G, Pingguan-Murphy B, Akbar SA. Modulation of osteoblast behavior on nanopatterned yttria-stabilized zirconia surfaces. Journal of the Mechanical Behavior of Biomedical Materials 2017; 68: 26–31. doi: 10.1016/j.jmbbm.2017.01.028
[26]Bergemann C, Duske K, Nebe JB, et al. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. Journal of Materials Science: Materials in Medicine 2015; 26: 26. doi: 10.1007/s10856-014-5350-x
[27]Cadafalch Gazquez G, Chen H, Veldhuis SA, et al. Flexible yttrium-stabilized zirconia nanofibers offer bioactive cues for osteogenic differentiation of human mesenchymal stromal cells. ACS Nano 2016; 10(6): 5789–5799. doi: 10.1021/acsnano.5b08005
[28]Frandsen CJ, Brammer KS, Noh K, et al. Tantalum coating on TiO2 nanotubes induces superior rate of matrix mineralization and osteofunctionality in human osteoblasts. Materials Science and Engineering: C 2014; 37: 332–341. doi: 10.1016/j.msec.2014.01.014
[29]Dong W, Cogbill A, Zhang T, et al. Multifunctional, catalytic nanowire membranes and the membrane-based 3D devices. The Journal of Physical Chemistry: B 2006; 110(34): 16819–16822. doi: 10.1021/jp0637633
[30]Dong W, Zhang T, Epstein J, et al. Multifunctional nanowire bioscaffolds on titanium. Chemistry of Materials 2007; 19(18): 4454–4459. doi: 10.1021/cm070845a
[31]Yuan ZY, Zhang XB, Su BL. Moderate hydrothermal synthesis of potassium titanate nanowires. Applied Physics A—Materials Science & Processing 2004; 78(7): 1063–1066. doi: 10.1007/s00339-003-2165-x
[32]Wang J, Yu Y, Li S, et al. Doping behavior of Zr4+ Ions in Zr4+-doped TiO2 nanoparticles. The Journal of Physical Chemistry: C 2013; 117(51): 27120–27126. doi: 10.1021/jp407662d
[33]Alizadeh A, Moztarzadeh F, Ostad SN, et al. Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artificial Cells, Nanomedicine, and Biotechnology 2016; 44(1): 66–73. doi: 10.3109/21691401.2014.909825
[34]Sidiqa AN, Hardiansyah A, Chaldun ER, Endro H. Preparation and characterization of zirconium oxide-doped hydroxyapatite. Key Engineering Materials 2019; 829: 54–59. doi: 10.4028/www.scientific.net/kem.829.54
[35]Wang X, Liu SJ, Qi YM, et al. Behavior of potassium titanate whisker in simulated body fluid. Materials Letters 2014; 135: 139–42. doi: 10.1016/j.matlet.2014.07.145
[36]Kokubo T, Yamaguchi S. Novel bioactive titanate layers formed on Ti metal and its alloys by chemical treatments. Materials 2009; 3(1): 48–63. doi: 10.3390/ma3010048
Copyright (c) 2021 Parker Cole, Yang Tian, Savannah Thornburgh, Mary Malloy, Lauren Roeder, Micah Maulding, Yang Huang, Z. Ryan Tian

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it for any purpose, even commercially, under the condition that the authors are given credit. With this license, authors hold the copyright.