Quantum chemical simulation of epirubicin interaction with fullerene and carbon graphene-like plane
Abstract
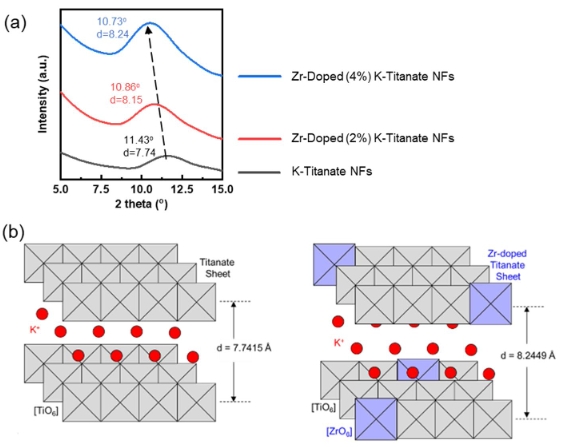
Creation of new “targeted delivery” drugs is one of priority areas of pharmacology and is especially true for oncology. Medicinal substances, in particular those of anthracycline series, immobilized on the surface of nanosized carriers for the targeted delivery of drugs to target organs or target tissues, allow creating an optimal concentration of the drug in the area of therapeutic effect. The latter significantly reduces systemic toxicity by decreasing the total dose and longer retention in the lesion, as well as increasing the solubility and bioavailability of drugs. Ones of promising drug delivery nanosystems are carbon materials, in particular, fullerene (C60) and pristine or modified graphene. The feature of carbon systems, in contrast to organic and dielectric transport systems, is their high conductivity and the dependence of the interaction energy between atoms of transporters and drugs on their charge state. To date, the specifics of the interaction of epirubicin with a graphene-like plane (GP) and fullerene at the atomic level remain poorly understood. Therefore, the energy parameters of the interaction of GP and C60 with epirubicin in various protolytic forms, which reveal at different pH values of the aqueous medium, were studied using quantum chemistry methods. Calculations were carried out using the MOPAC2016 program and the PM6-D3H4X method, in which, along with hydrogen bonds, the dispersion interactions are taken into account. Based on the analysis of the results of quantum chemical studies, the thermodynamic probability of the epirubicin adsorption process on GP is predicted in the entire pH range of the aqueous medium, as evidenced by the negative values of interaction enthalpies in all four cases. It has been found that epirubicin (protonated form) will have the greatest adsorption both on the graphene plane (−209.1 kJ/mol) and upon interaction with the fullerene molecule (−121.3 kJ/mol).
References
[1]Menna P, Gonzalez Paz O, Chello M, et al. Anthracycline cardiotoxicity. Expert Opinion on Drug Safety. 2011; 11(sup1): S21-S36. doi: 10.1517/14740338.2011.589834
[2]Becker MMC, Arruda GFA, Berenguer DRF, et al. Anthracycline cardiotoxicity: current methods of diagnosis and possible role of 18F-FDG PET/CT as a new biomarker. Cardio-Oncology. 2023; 9(1). doi: 10.1186/s40959-023-00161-6
[3]Huang J, Wu R, Chen L, et al. Understanding Anthracycline Cardiotoxicity From Mitochondrial Aspect. Frontiers in Pharmacology. 2022; 13. doi: 10.3389/fphar.2022.811406
[4]Russo M, Della Sala A, Tocchetti CG, et al. Metabolic Aspects of Anthracycline Cardiotoxicity. Current Treatment Options in Oncology. 2021; 22(2). doi: 10.1007/s11864-020-00812-1
[5]Sawicki KT, Sala V, Prever L, et al. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annual Review of Pharmacology and Toxicology. 2021; 61(1): 309-332. doi: 10.1146/annurev-pharmtox-030620-104842
[6]Cappetta D, Rossi F, Piegari E, et al. Doxorubicin targets multiple players: A new view of an old problem. Pharmacological Research. 2018; 127: 4-14. doi: 10.1016/j.phrs.2017.03.016
[7]McGowan JV, Chung R, Maulik A, et al. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovascular Drugs and Therapy. 2017; 31(1): 63-75. doi: 10.1007/s10557-016-6711-0
[8]Zhu H, Sarkar S, Scott L, et al. Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress. Reactive Oxygen Species. Published online May 2016: 189-198. doi: 10.20455/ros.2016.835
[9]Valcovici M, Andrica F, Serban C, et al. Cardiotoxicity of anthracycline therapy: current perspectives. Archives of Medical Science. 2016; 2: 428-435. doi: 10.5114/aoms.2016.59270
[10]Pourmadadi M, Farokh A, Rahmani E, et al. Polyacrylic acid mediated targeted drug delivery nano-systems: A review. Journal of Drug Delivery Science and Technology. 2023; 80: 104169. doi: 10.1016/j.jddst.2023.104169
[11]Lu Y, Shi Y, Wu Q, et al. An Overview of Drug Delivery Nanosystems for Sepsis-Related Liver Injury Treatment. International Journal of Nanomedicine. 2023; 18: 765-779. doi: 10.2147/ijn.s394802
[12]Iravani S, Varma RS. Advanced Drug Delivery Micro- and Nanosystems for Cardiovascular Diseases. Molecules. 2022; 27(18): 5843. doi: 10.3390/molecules27185843
[13]Cho K, Wang X, Nie S, et al. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clinical Cancer Research. 2008; 14(5): 1310-1316. doi: 10.1158/1078-0432.ccr-07-1441
[14]Liu R, Luo C, Pang Z, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chinese Chemical Letters. 2023; 34(2): 107518. doi: 10.1016/j.cclet.2022.05.032
[15]Fabozzi A, Della Sala F, di Gennaro M, et al. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab on a Chip. 2023; 23(5): 1389-1409. doi: 10.1039/d2lc00933a
[16]Hosseini SM, Mohammadnejad J, Najafi-Taher R, et al. Multifunctional Carbon-Based Nanoparticles: Theranostic Applications in Cancer Therapy and Diagnosis. ACS Applied Bio Materials. 2023; 6(4): 1323-1338. doi: 10.1021/acsabm.2c01000
[17]Chen L, Hong W, Duan S, et al. Graphene quantum dots mediated magnetic chitosan drug delivery nanosystems for targeting synergistic photothermal-chemotherapy of hepatocellular carcinoma. Cancer Biology & Therapy. 2022; 23(1): 281-293. doi: 10.1080/15384047.2022.2054249
[18]Jampilek J, Kralova K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials. 2021; 14(5): 1059. doi: 10.3390/ma14051059
[19]Anilkumar P, Lu F, Cao L, et al. Fullerenes for Applications in Biology and Medicine. Current Medicinal Chemistry. 2011; 18(14): 2045-2059. doi: 10.2174/092986711795656225
[20]Axet MR, Dechy-Cabaret O, Durand J, et al. Coordination chemistry on carbon surfaces. Coordination Chemistry Reviews. 2016; 308: 236-345. doi: 10.1016/j.ccr.2015.06.005
[21]Grebinyk A, Prylutska S, Chepurna O, et al. Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex. Nanomaterials. 2019; 9(11): 1540. doi: 10.3390/nano9111540
[22]Vovusha H, Banerjee D, Yadav MK, et al. Binding Characteristics of Anticancer Drug Doxorubicin with Two-Dimensional Graphene and Graphene Oxide: Insights from Density Functional Theory Calculations and Fluorescence Spectroscopy. The Journal of Physical Chemistry C. 2018; 122(36): 21031-21038. doi: 10.1021/acs.jpcc.8b04496
[23]Petit K, Suwalsky M, Colina JR, et al. Toxic effects of the anticancer drug epirubicin in vitro assayed in human erythrocytes. Toxicology in Vitro. 2020; 68: 104964. doi: 10.1016/j.tiv.2020.104964
[24]Luiz MT, Dutra JAP, Di Filippo LD, et al. Epirubicin: Biological Properties, Analytical Methods, and Drug Delivery Nanosystems. Critical Reviews in Analytical Chemistry. 2021; 53(5): 1080-1093. doi: 10.1080/10408347.2021.2007469
[25]Launchbury AP, Habboubi N. Epirubicin and doxorubicin: a comparison of their characteristics, therapeutic activity and toxicity. Cancer Treatment Reviews. 1993; 19(3): 197-228. doi: 10.1016/0305-7372(93)90036-Q
[26]Anilanmert B, Arioz Ozdemir F, Erdinc N, et al. Potentiometric determination of the dissociation constants of epirubicin HCl and irinotecan HCl. Mendeleev Communications. 2006; 16(2): 97-98. doi: 10.1070/mc2006v016n02abeh002234
[27]Zhu S, Yan L, Ji X, et al. Conformational diversity of anthracycline anticancer antibiotics: A density functional theory calculation. Journal of Molecular Structure: THEOCHEM. 2010; 951(1-3): 60-68. doi: 10.1016/j.theochem.2010.04.008
[28]Samide A, Tutunaru B, Varut RM, et al. Interactions of Some Chemotherapeutic Agents as Epirubicin, Gemcitabine and Paclitaxel in Multicomponent Systems Based on Orange Essential Oil. Pharmaceuticals. 2021; 14(7): 619. doi: 10.3390/ph14070619
[29]Lombardi P, Animati F, Cipollone A, et al. Synthesis and conformational preference of novel 8-fluoroanthracyclines. Acta Biochimica Polonica. 1995; 42(4): 433-444. doi: 10.18388/abp.1995_4897
[30]Krätschmer W, Lamb LD, Fostiropoulos K, et al. Solid C60: a new form of carbon. Nature. 1990; 347(6291): 354-358. doi: 10.1038/347354a0
[31]Chan B, Kawashima Y, Katouda M, et al. From C60 to Infinity: Large-Scale Quantum Chemistry Calculations of the Heats of Formation of Higher Fullerenes. Journal of the American Chemical Society. 2016; 138(4): 1420-1429. doi: 10.1021/jacs.5b12518
[32]Cherniuk OA, Demianenko EM, Terets MI, et al. Study of the mechanism of influence of carbon nanotubes surface chemistry on the mechanical properties of fiberglass. Applied Nanoscience. 2020; 10(12): 4797-4807. doi: 10.1007/s13204-020-01448-1
[33]Stewart JJP. MOPAC2016. Stewart Computational Chemistry. Colorado Springs, CO, USA; 2016.
[34]Stewart JJP. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. Journal of Molecular Modeling. 2007; 13(12): 1173-1213. doi: 10.1007/s00894-007-0233-4
[35]Řezáč J, Hobza P. Advanced Corrections of Hydrogen Bonding and Dispersion for Semiempirical Quantum Mechanical Methods. Journal of Chemical Theory and Computation. 2011; 8(1): 141-151. doi: 10.1021/ct200751e
[36]Řezáč J, Riley KE, Hobza P. Benchmark Calculations of Noncovalent Interactions of Halogenated Molecules. Journal of Chemical Theory and Computation. 2012; 8(11): 4285-4292. doi: 10.1021/ct300647k
Copyright (c) 2024 B. M. Gorelov, O. V. Khora, E. M. Demianenko, N. A. Havryliuk, A. G. Grebenyuk, V. V. Lobanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it for any purpose, even commercially, under the condition that the authors are given credit. With this license, authors hold the copyright.